From battlefield to home: a mobile platform for assessing brain health
Introduction and overview
In addition to regulating breathing, heart rate, and blood pressure, the brain receives information from the environment, interprets this information, and guides appropriate responses to these stimuli. From an evolutionary point of view, an organism’s ability to effectively process external information is advantageous because it facilitates survival (1). In prehistoric times, the ability to react quickly to visual stimuli could make the difference between a successful hunt and starvation. In the modern era, efficient processing of external information has implications for tasks as diverse as identifying the best moment to swing a baseball bat, being able to distinguish friend from foe on the battlefield, and being able to recognize and respond to traffic signals. Cognitive efficiency refers to how quickly and accurately one can process information, and this aspect of brain function has far-reaching implications for well-being throughout the life span and into old age (2-4). The importance of assessing and maintaining cognitive efficiency has led to development of tools to measure various aspects of brain health and function (5).
Historically, cognitive testing has been conducted in office-based settings by specially-trained professionals such as neuropsychologists. Cognitive batteries that are administered in these settings include intelligence tests, finger tapping tests, trail making tests, coding tests, letter-number sequencing tests, verbal learning tasks, and block design tests. These tests evaluate different aspects of brain function, including cognitive efficiency or processing speed, spatial processing, visual scanning and attention, immediate recall, short-term memory, working memory, language, attention/concentration, executive function, and visual-spatial discrimination (6). The variety and complexity of brain functions that are assessed by cognitive testing batteries hint at the many ways that deficits in these functions can unfavorably impact daily function. For example, an injured student athlete’s grades may decline, a cognitively impaired older adult may lose keys or leave the stove on, and an injured soldier may put himself and his unit at risk.
The emergence of mHealth offers an opportunity for radical changes in how we assess cognitive or brain health, and this report explores four considerations related to this paradigm shift: (I) limitations of traditional approaches to cognitive testing; (II) opportunities for mobile assessment of brain health; (III) mobile platforms for patient-centered cognitive assessment; and (IV) re-thinking data and outcomes. These considerations reveal three broad themes related to the evolution of cognitive efficiency testing: A shift from disease diagnosis in the office setting to mobile tracking of health and wellness in any setting; the strength of computer-based measures and their role in facilitating development of new computational methods, and the use of cognitive testing to inform on individual-level outcomes over time rather than dichotomous metrics at a single point in time.
Limitations of traditional approaches to cognitive testing
By definition, identification of a cognitive deficit is required before appropriate interventions can be implemented. For this purpose, traditional paper and pencil testing is reliable, valid, and has diagnostic value—all important features for clinical application. However, traditional approaches to assessment of brain health also come with a number of important limitations. These tests are time-intensive for both testers and patients; special training and testing areas are needed; they are expensive; it can be difficult to get short-term evaluations because access to neuropsychological services is limited; there are learning effects that can’t be mitigated by alternate forms of the tests, and the tests were not designed to be patient-centered tools or to assess how people function in community-based settings (7,8).
In addition to these issues, there are important limitations related to the nature of the data, how they are collected, and how these factors interact to impact usability (9). For example, because paper and pencil tests are not computerized, factors related to how these tests are administered can impact scoring across testing environments. The tests do not permit export of raw or summary data in a manner that facilitates data analysis or integration with patients’ electronic health records. Test batteries frequently yield simple summary scores on various sub-tests, a system that does not offer insight into complex response patterns that may provide important insight on the presence or origin of various aspects of cognitive deficit. Finally, these testing modalities focus heavily on data collection at a single point in time, and comparisons of these cross-sectional measures to population-based norms. Thus, they are not designed to track individuals’ cognitive efficiency over time, nor are they designed to put patient data in patients’ hands where this information can be acted upon when a meaningful change in performance occurs.
Opportunities for mobile assessment of brain health
In recent years there has been a call for development and broad implementation of computerized cognitive testing. This need has been highlighted by stakeholders including drug developers, federal agencies that sponsor research focused on cognitive outcomes, and from clinicians who wish to move toward testing strategies that provide greater access to cognitive data in a manner that offers faster, more detailed information without sacrificing quality or increasing patient burden (10,11). In addition to these stakeholders, patients and caregivers are also developing higher expectations concerning the quality of, and access to their own health-related data (12,13). Mobile cognitive testing responds to stakeholder demands, offering a number of advantages over traditional methods, including considerations related to ease of administration and access to data.
Beyond these obvious advantages, mobile cognitive testing is patient-centered, allowing patients unprecedented access and insight into their own cognitive efficiency at a single point in time as well as understanding of patterns of change over time. The value of this information is not limited to patients. The vast majority of care for people with chronic disease comes from informal caregivers—most often from adult children and elderly spouses (14). The availability of a mobile platform that caregivers can use to assess a care recipient’s cognitive efficiency may offer new opportunities for caregivers to reliably track cognitive change over time, thereby enabling them to be more effective caregivers. Meeting these needs is consistent with federal priorities concerning the need to help “family caregivers to continue to provide care while maintaining their own health and well-being.” (15).
The variety of settings in which cognitive deficits can impact day to day function—the baseball field, the battle field, nursing homes, and community-based residences—reflect the value of having mobile cognitive assessment tools that can be used effectively in diverse settings. These technologies can also be used repeatedly over time in a manner that informs on clinically meaningful trends, and that puts actionable information directly in the hands of consumers.
Finally, the limitations of traditional cognitive testing highlight the ways in which a new generation of mobile cognitive efficiency testing strategies can meet evolving patient needs. For example, assessment of cognitive efficiency at during primary care visits would establish individual baseline, allowing highly sensitive assessments of changes that might occur due to a sports injury, depression, or age-related dementia. Mobile tracking could also enable measurement-based care. Underscoring the desire to base healthcare on objectives measures, the Kennedy Forum recently issued a national call to expand the practice of measurement-based care from medical and surgical fields to behavioral health (16).
Mobile platforms for patient-centered cognitive assessment
Advances in technology, improved health literacy, and the independence that has been fostered by mobile technologies have all contributed to a shift in patients’ expectations of their interactions with the healthcare system and their own health information. Patients—particularly younger patients—expect to access their health data and health care providers in ways that were unthinkable 15 years ago. Many primary care practices offer portals that allow patients to make appointments online, to access their laboratory results, and to request prescriptions refills. Policy-driven incentives encouraging primary care providers to adopt electronic health records, combined with ongoing efforts to enhance patient access with mobile technology reflect broader trends that recognize the importance of technology-enhanced patient-centered care (17). Patients have developed a new set of expectations concerning access to their own health data, and there is a new sense of autonomy among patients that reflects the desire to have a greater degree of data-driven control over their health and wellness (18).
It is against this backdrop that traditional strategies to assess cognitive performance should be re-evaluated. If cognitive testing can be conducted reliably outside of an office setting, it is reasonable to expect that these testing strategies should be taken into the field—taken to patients—rather than continuing to expect patients to come to the office. A key principle of “patient centered care” is the idea that patients are the best source of information about how well their health care providers are meeting their needs, and those patient perceptions about their healthcare delivery correlate with both health outcomes and satisfaction with care (19).
Although age-related cognitive decline is not the only setting in which mobile brain health technologies provide benefit to patients and families, this setting provides a useful framework to think about the value of these strategies. Among older adults, there are numerous non-office settings where cognitive testing could provide useful information to both formal and informal caregivers. These have direct application to patient-centered care because it is well-established, for example, that older adults have a strong preference to remain independent in their homes as long as possible. Such preferences, along with the recognized cost advantages of providing community-based—as opposed to institutional—care for frail seniors, is at the root of a shift toward development of systems for community-based provision of long term care supports and services. A key element of care plans that are implemented in the community is a clear understanding of care recipients’ cognitive status. The frailty of this population, along with a focus on home-based care reflect the value of mobile assessment tools that can provide integrated care teams with information on cognitive status over extended periods of time in a manner that informs on diverse aspects of care for growing numbers of seniors.
The value of this information can be interpreted in the context of the diversity of settings in which older adults reside, and in which tracking of their cognitive efficiency would be useful not only to them, but also to both formal and informal caregivers. For older adults who use nursing home services, ongoing assessment of cognitive efficiency could inform directly on various aspects of institutional care, and this information could be readily collected in this care setting using mobile platforms, and it would be available not only clinicians, but also to patients and family members. Intermediate between community/home-based residential settings and institutional care are assisted living settings in which seniors receive a limited set of health services. Like nursing home settings, care teams in these residential settings could benefit from easy access to reliable data on seniors’ cognitive efficiency. The ability of mobile technologies to dovetail with electronic health records would further enhance continuity of care for frail older adults who receive care from numerous specialists who practice in these diverse care settings.
Re-thinking data and outcomes
In recent years, there has been a tremendous increase in awareness of the role that “big data” can play in clinical decision-making, including how it can be used to personalize cognitive health (20,21). There is parallel interest in the idea that objective data should be at the foundation of individualized decisions about health, and that generation of, and access to clinical data should extend beyond the doctor’s office; it should be tailored to the needs of individual patients, it should provide insight on patients’ longitudinal health trends, the information should available to patients and their families on demand, and data should be available using technologies that are chosen by consumers (22).
Among the drivers of the increased emphasis on collection of individualized data for cognitive assessment in particular is the aging of the U.S. population, sometimes called “the graying of America” or the “silver tsunami”. Growing numbers of older adults have resulted in a marked increase in the burden of Alzheimer’s disease and other dementias. These burdens not only impact patients, but they also have unfavorable effects on informal caregivers as well as the formal healthcare system.
New mobile technologies capture, export, and facilitate analysis of computerized cognitive data in a manner that enables use of all data that are collected by these technologies, not just summary scores. Why is this important for cognitive testing? Unlike many biological determinations (e.g., blood glucose or cholesterol) where a single threshold measure can unambiguously define the presence or absence of a disease or risk state, cognitive deficits can be subtle, and they can occur in multiple areas of brain function. Assessment of the presence or absence of a cognitive health condition that requires intervention may require many tests that evaluate multiple brain functions, often using a single summary score that is supposed to capture a multitude of complex patterns and functions.
Historically, cognitive testing scores are collapsed so that cognitive status is presented as binary (impaired/not impaired) or ordinal (normal/mild impairment/moderate impairment/severe impairment). This framework has important limitations. It is constrained by the maxim values that are dictated by the sum of scores on component tests. Pooling of sub-scores can obscure profound cognitive impairment on one subtest while still showing a favorable overall score. A third limitation involves the assumption that a single overall score offers the greatest clinical utility and that patterns of fluctuation over the course of many trials are of little or no value to cognitive assessment or care planning (23).
We believe that efforts to optimize the richness of computerized cognitive testing data must fully utilize all trial-by-trial data that are offered by these technologies because of the tremendous insight that this highly granular information can provide. These strategies offer an opportunity to depart from a traditional framework that relies on a single set of summary scores to one in which new computational methods can capitalize on many thousands of data points to provide insight on subtle changes in cognitive efficiency over time. The growing use of mobile cognitive assessment technologies will only enhance the impact of these efforts because of their ability to facilitate access to this information on the part of patients and families.
It is helpful to use a specific example to illustrate some of these concepts. Simple reaction time (SRT) assesses psychomotor speed—often in response to a visual stimulus, and the test often involves between 20 and 50 trials depending on the tool or instrument. Historically, SRT summary scores have been used as a means to describe an individual’s global performance on this subtest at a single point in time without regard to quantifying the shape of the curve that is generated by performance on each trial, and without appreciable attention to how response patterns may change over time. We propose a new focus that uses all the data that are available from newer mobile technologies to provide both a more granular view of an individual’s cognitive efficiency at a given point in time, and to help quantify meaningful changes over time.
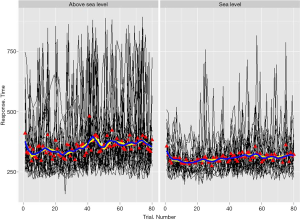
This new focus can potentially unlock new applications of cognitive testing data in a quantitative and clinically relevant framework that is consistent with evolving expectations of patient-centered care. These methods could reveal previously-unidentified deficits and perhaps the etiology of some forms of cognitive dysfunction. An example of this strategy is presented in Figure 1. This figure shows SRT data from young adults at sea level, and the same adults at extreme altitude where their cognitive efficiency was greatly diminished due to hypoxia. These data, which were collected with a hand-held mobile cognitive assessment instrument, reveal significant differences in data patterns between the two groups, with hypoxic individuals’ SRT data being significantly more unstable than their uninjured counterparts. Examination of simple means and standard deviations do very little to fully utilize the richness of these data. We continue to develop these and other new computational methods to meet the growing expectations of patients and caregivers who are coming to expect more than a simple “yes or no” concerning questions about their health status.
Conclusions
Mobile platforms for computerized cognitive testing offer new opportunities to put actionable health information in the hands of consumers, to develop novel computational strategies that fully leverage large amounts of highly detailed cognitive efficiency data, and to meet the needs of diverse populations in a fully patient-centered framework.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Survival – mind and brain. Available online: (accessed June 3, 2016).https://thepsychologist.bps.org.uk/volume-24/edition-1/survival-%E2%80%93-mind-and-brain
- Hoffman B. Cognitive efficiency: A conceptual and methodological comparison. Learning and Instruction 2012;22:133-44. [Crossref]
- Rypma B, Berger JS, Prabhakaran V, et al. Neural correlates of cognitive efficiency. Neuroimage 2006;33:969-79. [Crossref] [PubMed]
- Derakshan N, Eysenck MW. Anxiety, processing efficiency, and cognitive performance new developments from attentional control theory. European Psychologist 2009;14:168-76. [Crossref]
- Cullen B, O'Neill B, Evans JJ, et al. A review of screening tests for cognitive impairment. J Neurol Neurosurg Psychiatry 2007;78:790-9. [Crossref] [PubMed]
- Harvey PD. Clinical applications of neuropsychological assessment. Dialogues Clin Neurosci 2012;14:91-9. [PubMed]
- Sbordone RJ. Limitations of neuropsychological testing to predict the cognitive and behavioral functioning of persons with brain injury in real-world settings. NeuroRehabilitation 2001;16:199-201. [PubMed]
- Johnstone B, Frank RG. Neuropsychological assessment in rehabilitation: Current limitations and applications. NeuroRehabilitation 1995;5:75-86. [Crossref] [PubMed]
- Wesnes KA. Moving beyond the pros and cons of automating cognitive testing in pathological aging and dementia: the case for equal opportunity. Alzheimers Res Ther 2014;6:58. [Crossref] [PubMed]
- Wild K, Howieson D, Webbe F, et al. Status of computerized cognitive testing in aging: a systematic review. Alzheimers Dement 2008;4:428-37. [Crossref] [PubMed]
- Bauer RM, Iverson GL, Cernich AN, et al. Computerized neuropsychological assessment devices: joint position paper of the American Academy of Clinical Neuropsychology and the National Academy of Neuropsychology. Arch Clin Neuropsychol 2012;27:362-73. [Crossref] [PubMed]
- Caine K, Tierney WM. Point and counterpoint: patient control of access to data in their electronic health records. J Gen Intern Med 2015;30 Suppl 1:S38-41. [Crossref] [PubMed]
- Mandl KD, Kohane IS. Time for a patient-driven health information economy? N Engl J Med 2016;374:205-8. [Crossref] [PubMed]
- 2015 Alzheimer’s Disease Facts and Figures. Available online: (accessed June 3, 2016).https://www.alz.org/facts/downloads/facts_figures_2015.pdf
- National plan to address Alzheimer's disease: 2015 update. Available online: (accessed June 3, 2016).https://aspe.hhs.gov/national-plan-address-alzheimers-disease-2015-update#strategy3
- A National Call for Measurement-Based Care. Available online: (accessed June 13, 2016).https://www.thekennedyforum.org/news/measurement-based-care-issue-brief
- Health IT Legislation and Regulations. Available online: (accessed June 3, 2016).https://www.healthit.gov/policy-researchers-implementers/health-it-legislation-and-regulations
- Available online: (accessed June 3, 2016).https://www.healthit.gov/patients-families/your-health-data
- Epstein RM, Street RL Jr. The values and value of patient-centered care. Ann Fam Med 2011;9:100-3. [Crossref] [PubMed]
- Geerts H, Dacks PA, Devanarayan V, et al. From big data to smart data in Alzheimer's disease. The brain health modeling initiative to foster actionable knowledge. Alzheimers Dement 2016. [Epub ahead of print]. [Crossref] [PubMed]
- Cheung MW, Jak S. Analyzing big data in psychology: a split/analyze/meta-analyze approach. Front Psychol 2016;7:738. [Crossref] [PubMed]
- Maglaveras N, Kilintzis V, Koutkias V, et al. Integrated Care and Connected Health Approaches Leveraging Personalised Health through Big Data Analytics. Stud Health Technol Inform 2016;224:117-22. [PubMed]
- Wang L, Zhang Z, McArdle JJ, et al. Investigating ceiling effects in longitudinal data analysis. Multivariate Behav Res 2009;43:476-496. [Crossref] [PubMed]
Cite this article as: Resnick HE, Lathan CE. From battlefield to home: a mobile platform for assessing brain health. mHealth 2016;2:30.


