Pilot randomized trial of MOMENT, a motivational counseling-plus-ecological momentary intervention to reduce marijuana use in youth
Introduction
By 12th grade, 44.5% of adolescents have tried marijuana, 12% have used daily, and 6% report current daily use (1). Marijuana is the leading drug of abuse among adolescents aged 12–17 years, contributing to 87% of their admissions for substance use treatment (2). Adolescents are at considerably higher risk for cannabis use disorder (CUD) than both younger and older adults (3), although young adults have a higher prevalence of daily use (e.g., 7.8% in 19–22 years old) (4). Heavy exposure to marijuana during brain development (into the late 20s) has adverse effects on white and gray matter in regions associated with cognition, sensitivity to social influence, addiction-related processes (e.g., reward), and psychiatric disorders, and is associated with other substance use, mental health problems, and poor educational, occupational, financial, and other social outcomes (5-12). As public discourse and state laws have shifted toward greater acceptability of marijuana, perceived risk of regular use among youth has declined to an all-time low (1,4), raising concerns about future increases in regular marijuana use in this vulnerable population. Most affected youth are not in treatment programs, but may be seen in primary care, where resources and expertise to address problematic marijuana use in young patients are limited.
Screening, Brief Intervention, and Referral to Treatment (SBIRT) is a structured approach to addressing substance use in primary care. SBIRT guidelines often include recommendations for brief motivational interviewing (MI) when problematic substance use is identified (13,14). Although motivational interventions for youth can be effective in reducing marijuana use and, to a lesser extent, achieving abstinence, evidence is mixed (15-17). Effects following treatment may not be sustained, reflecting that change is a process needing ongoing work in daily life. Integrating motivational interventions with other treatment modalities (e.g., cognitive behavior therapy) may produce better or longer-term improvements in outcomes (18), but multiple-session intensive interventions may not be practical in primary care.
Ecological momentary interventions (EMIs) (19) provide an alternative means of extending and enhancing clinic-based interventions. Using mobile technology (e.g., smartphones), EMIs deliver interventions in daily life and thus can be responsive to emotional and social experiences as they are occurring. EMIs can encourage practice of new skills and behaviors in real-life contexts, theorized to be necessary for behavior change (20). EMIs integrated with face-to-face counseling may be more effective than stand-alone EMIs (21). We developed Momentary Self-Monitoring and Feedback + Motivational Enhancement Therapy (MOMENT), an intervention to reduce marijuana use that combines brief motivational enhancement therapy (MET) provided in clinic followed by EMI (self-monitoring and messaging responsive to context and behaviors) delivered via mobile technology (22,23). A small single-group pilot suggested that MOMENT is feasible and acceptable to adolescent and young adult primary care patients who report frequent marijuana use (22). If effective, MOMENT could facilitate greater integration of marijuana use treatment into primary care SBIRT to reach youth not currently served by existing treatment paradigms.
The primary aim of this pilot trial was to evaluate the feasibility of implementing a larger-scale randomized trial of the MOMENT intervention in primary care settings serving adolescents and young adults. The specific primary objectives were: (I) to determine recruitment, retention, and response rates during the baseline, intervention, and 3-month follow-up phases of the study, and (II) to further evaluate the acceptability of the intervention. The secondary objectives were: (I) to assess safety concerns and technical issues and (II) to measure key variables and explore key relationships hypothesized to be important in evaluating intervention efficacy in a future trial.
Methods
Study design
We conducted a pilot parallel-group trial with 1:1 assignment to three arms via simple randomization. All participants were offered two MET sessions; groups differed by whether they proceeded to mobile self-monitoring with feedback messages (MOMENT), mobile self-monitoring alone (No-messages), or no further treatment (MET-only). Because we were identifying youth with frequent marijuana use, we chose to offer active treatment to all participants rather than include a standard-of-care control.
Participants and procedures
We recruited from five clinics providing primary care to adolescents and young adult patients of an urban children’s hospital. The recruitment sites included three clinics on the hospital campus —an adolescent/young medical clinic, a pediatric primary care clinic, and a clinic for teen parents and their young children—serving patients 40–50% black or African American, 12–35% Hispanic/Latino, and majority low-income. The other two clinics, an adolescent/young adult medical clinic and a pediatric primary care clinic, are based in a hospital-operated community health center serving patients primarily of Hispanic/Latino ethnicity and low-income. Eligibility criteria included age 15–24 years, marijuana use ≥3 times/week, ability to read/understand English, and availability for the 4-month study. Patients were excluded if they were medically or emotionally unstable, intoxicated, or otherwise unable to consent; reported past-30-day heavy/dangerous use of substances other than marijuana; or were living with a participant. Initially, individuals without their own smartphone were excluded. To expand enrollment, we revised eligibility to permit individuals age 18 and older use of a study smartphone. The second change to eligibility, excluding parenting youth, followed safety concerns for a participant’s children. The hospital institutional review board approval waived parental permission and the National Institute on Drug Abuse provided a Certificate of Confidentiality.
Between May, 2013 and January, 2016, during routine care, clinicians introduced the study to 15-to-24-year-old patients who used marijuana. Additionally, a Clinic Research Coordinator contacted age-eligible patients who had indicated interest in research and patients could self-refer. A research assistant (RA) screened interested patients and obtained informed consent from those eligible using a video to illustrate study procedures.
Participants provided baseline data on a computer tablet survey and a 30-day timeline follow-back (TLFB) calendar, and received training on the ecological momentary assessment (EMA) phone application (app; mEMA, Ilumivu, Inc.). For one week, participants received 4–6 signals/day at random times, prompting report of marijuana-related momentary states, contexts, and behavior. Participants also completed self-scheduled daily diaries, summarizing marijuana withdrawal symptoms, cigarette and marijuana use, and motivation to reduce or cease marijuana over the past 24 hours.
Participants then met with a study counselor in clinic for two 1-hour MET sessions separated by one week. In the first session (MET1), the counselor established rapport; discussed marijuana history, behaviors, reasons, and expectancies; elicited personal goals and values; and developed discrepancy between use and the values and goals. Participants identified triggers for use from lists of affective states and social contexts, and then selected their top 3 triggers. In the second session (MET2), participants received and discussed a personalized feedback report on their use. The counselor evoked motivation for reducing or ceasing marijuana use and helped participants to develop a change plan. One investigator (PJ Burke) trained two study counselors in MI through didactic sessions and mock interviews. The trainer monitored fidelity to MI principles and the MET manual (12 participants’ sessions according to a pre-determined schedule) by: (I) the counselor and the trainer each rating the audio recorded sessions using an adapted Behaviour Change Counselling Index [BECCI (24); 14 items, 0 = not at all to 4 = a great extent; a =0.92]; (II) the trainer rating each session using the Global Ratings and Behavior counts of the Motivational Interviewing Treatment Integrity Code version 3.1.1 then 4.0 (25); and (III) the trainer meeting with the counselor to review the ratings and provide coaching. These coaching meetings enabled the trainer to provide ongoing feedback and qualitatively demonstrated that the counselors maintained high fidelity to MI and the manual. Median BECCI item scores from both trainer evaluations and counselor self-evaluations were all 3s (a good deal) or higher, indicating competence in brief MI counseling.
MOMENT arm
For participants assigned to MOMENT, the RA programmed the app to display motivational messages following momentary report of a personal top-3 trigger for use, marijuana desire, use, or effort to avoid use. Messages were developed with input from key informants (23). MOMENT participants completed reports and received messages for 2 weeks. The RA sent a check-in text message after 48–72 hours. For participants with a response rate of <70%, the message advised seeking technical help and reminded them to respond. We provided an app “cheat sheet” and a trouble-shooting guide accessible via secure online file-sharing.
No-messages arm
Participants in the No-messages arm completed 2 weeks of smartphone reports without messages. After assigning 15 participants to No-messages, we discontinued this arm to enrich assignment to MOMENT and MET-only for the remainder of the funding period.
MET-only arm
Participants in the MET-only arm did not receive any intervention following MET2.
At a visit 2 weeks after MET (at the end of any EMA/EMI), all participants were asked to recall marijuana and other substance use and provide study feedback. In a visit three months later, participants completed a follow-up survey and 30-day TLFB calendar, then one week of EMA. At the final visit we elicited study feedback. After the first 9 months, to optimize retention, we allowed participants to do the post-intervention, 3-month follow-up, and final visits via mobile survey and call. We mailed phones to participants who chose the remote option and did not have an app-compatible phone. We offered participants remuneration (up to $175 total), initially following each phase and then, to improve retention, following each visit. Remuneration was graded over the study and commensurate with completion of study assessments, including study visits ($15–25) and EMA reports ($10–15 for responding to at least 50% of prompts and $20–25 for responding to at least 80% of prompts). Participants were not paid according to whether they used marijuana or whether they completed reports during the intervention phase (for participants in the No-messages and MOMENT arms).
Measures
Feasibility
We determined monthly recruitment and enrollment rates, and examined retention by visit, phase, and arm. We calculated momentary and daily response rates for each phase.
Acceptability
Following each MET session, participants indicated agreement on a 5-point Likert-type scale to 21 items (e.g., discussing my personal goals was helpful; the counselor respected me); one item assessed session quality overall (poor, fair, good, excellent). After the intervention and follow-up phases, we assessed acceptability of procedures (e.g., usability, likability, perceived effect, and burden; 15 items), and study usefulness overall (poor, fair, good, excellent), and asked participants to write in their most and least favorite parts of the study, what they would change, and any additional comments. On all four acceptability assessments, participants were invited to complete the statement, “As a result of taking part in this study, I….”.
Key variables
We examined response patterns and preliminarily tested relationships between key momentary variables for which there has been limited evaluation (22). Momentary marijuana desire was assessed by, “At the time of the signal, how strong was your desire to use marijuana?” (0, no desire to 9, very strong desire). Recent marijuana use was assessed by, “Since the last signal you answered, have you used marijuana?” (yes/no). Top-3 trigger context was determined by indicating a self-identified top-3 trigger for use at the signal, determined from responses selected from 8 locations (e.g., home, friend’s house), 11 companions (e.g., alone, with parents), 9 main activities (e.g., work or chores, hanging out), and 10 affective states (5 positive, e.g., happy, confident, and 5 negative, e.g., bored, stressed) (22).
Baseline and 3-month follow-up past-30-day percent days abstinent (PDA) was calculated from the TLFB. Problems with substance use, in general and with marijuana, were assessed with the 17-item (yes/no) Problem-Oriented Screening Instrument for Teenagers (POSIT) Substance Use/Abuse scale (26).
Sample characteristics
Sociodemographic and historical items were adapted from the Adolescent Diagnostic Interview (27) (Table 1). Responses to questions on use of marijuana and other drugs and associated problems were used to generate substance use disorder diagnoses.
Full table
Analysis
We computed descriptive statistics for recruitment, retention, and response rates, and responses on the feedback surveys. We conducted bivariate analyses (appropriate to variable distribution) to examine retention by baseline characteristics and arm.
To evaluate intervention effects on the key outcomes, we used linear or generalized mixed effects modeling, with repeated measures nested within participants, treating phases as fixed effects, and intercepts of individual trajectories as random effects. We modeled covariance structure as first-order autoregressive and used the Bayesian Information Criterion to identify the best fitting model for a given outcome distribution and corresponding link function. Models included arm, phase, and arm-by-phase interaction, and adjusted for age of initiating marijuana use ≥3 times/week (differed across arms). For momentary outcomes, we adjusted for within-phase average reports/day (to control for potential response rate bias) and percent reports of a top-3-trigger context (to control for variability attributable to level of exposure). To compare change in momentary marijuana use reports, we analyzed all data, then the subset of reports made within 6 hours following an EMI-targeted context or behavior to narrow the window of temporal precedence.
Results
Feasibility
Recruitment/enrollment
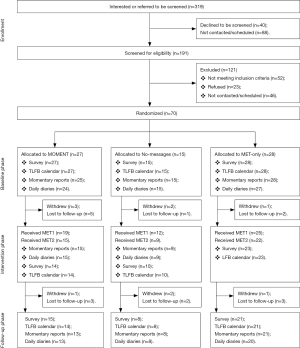
Of 319 patients expressing interest or referred for screening, 191 (59.9%) were screened [mean (M) =6/month] and 139 (73% of those screened) were eligible (Figure 1). Seventy (50%) were enrolled and randomized (M =2.2/month); 27 were assigned to MOMENT, 15 to No-messages, and 28 to MET-only. Enrollment was closed after 70 participants to provide time for follow-up and analysis during the project period.
Sample characteristics
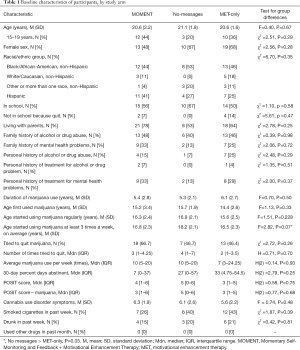
Table 1 shows participant baseline characteristics by arm. Participants were M (SD) =20.7 (1.9) years of age (36% adolescents aged 15–19 years), 60% female, and of diverse race/ethnicity. They reported using marijuana for median (Mdn) =5.6 years, with current use Mdn =9.5 times/week. More than one-half (56%) had tried to quit marijuana at least once (Mdn =2.5 times). All but 3 met criteria for CUD (2 in MET-only, 1 in No-messages). Participants assigned to No-messages vs. MET-only were significantly older when they started using marijuana at least 3 times/week (Mdn =18.2 vs. 16.5 years, U=126.5, P=0.03). We found no other significant differences in baseline characteristics between arms.
Retention
Sixty-six percent of the sample completed their assigned treatment (46/70) and 63% (44/70) completed at least one 3-month assessment. Most attrition occurred before MET1 [14/70 (20%); 10.7% MET-only, 20.0% No-messages, 29.6% MOMENT, χ2 =3.07, P=0.22]. Participants who dropped out vs. completed MET1 were more likely to have previously attempted to quit marijuana (85.7% vs. 46.4%, χ2 =6.97, P=0.008) and tended to have more CUD symptoms (Mdn =7.5 vs. 6, U=264.5, P=0.058); there were no other differences in baseline characteristics between those starting the treatment phase and those who dropped out.
Of the 56 who completed MET1, 46 (82%) completed their assigned treatment and 44 (79%) completed at least one 3-month assessment. Most frequent reasons for withdrawal were “can or already has cut back or quit on own”, difficulty scheduling study activities, and too many reports. There were no significant differences in baseline characteristics between participants completing MET1 who did vs. did not return at 3 months.
Response rates
Momentary response rates were Mdn [interquartile range (IQR) =63.5% (46.6–74.8%) at baseline and 57.1% (29.4–73.5%) at 3-month follow-up. Diary response rates were Mdn (IQR) =85.7% (71.4–100%) at baseline and 71.4% (57.1–87.5%) at follow-up. During the intervention phase (MOMENT and No-messages arms), response rates were Mdn (IQR) =35.1% (24.6–60.4%) of the momentary reports and 57.1% (39.3–85.2%) of the diaries. Response rates were similar across arms in each phase.
Acceptability
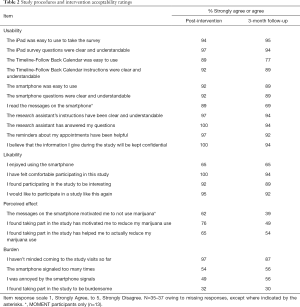
Participants rated overall quality of the MET sessions as excellent or good (MET1, 93%; MET2, 94%). In general, participants agreed or strongly agreed that the assessments and tools were easy to use and the questions were clear and understandable (Table 2). Nearly all participants completing the post-intervention feedback survey indicated that they found the study interesting and would participate in a similar study again. Two-thirds (65%) enjoyed using the smartphone. Although approximately one-half of respondents felt the smartphone signals were too frequent (54%) or annoying (49%), less than one-third (32%) found participation burdensome. Most found that participating motivated them to reduce marijuana use (76%) or helped them to actually reduce use (65%). Most participants assigned to MOMENT read the smartphone messages (89%) and felt that the messages motivated them not to use marijuana (62%). At study conclusion, participants gave similar feedback, although lower proportions perceived an effect of study participation on their marijuana use. Post-intervention, 91% rated overall study usefulness as “excellent” or “good”; at 3 months, 36% did so. There were no significant differences in acceptability by study arm.
Full table
Free text comments illustrated changes in motivation and behavior. After MET1, participants wrote statements consistent with preparing to change, e.g., that they were “better able to weigh the pros and cons of smoking marijuana,” had become “more driven towards pursuing my goals”, and felt “more confident” and “better equipped” to reduce use. After MET2, participants indicated increased confidence and commitment to change, e.g., they felt ready or had already begun to use less marijuana. Comments after the intervention phase referenced raised awareness of triggers and patterns of marijuana use (e.g., “[I] was able to notice trends of marijuana use habits and try to avoid them”). Many youths favored the counseling sessions, while others preferred the smartphone (“being able to keep track of how much I was using marijuana”, “how you get a motivational message when you’re alone in order to not be tempted to use marijuana”). Negative comments referred to too-frequent signals and repetitive messages.
Safety
No safety concerns arose directly from the intervention. A counselor breached confidentiality to clinicians per study protocol when a participant’s marijuana use raised concerns for her children’s well-being.
Technical issues
Owing to software malfunctions interfering with signaling and changes during updates, we elected not to continue with updates and thus required participants to use phones compatible with our version of the software. We loaned phones to 55 participants, with a return rate of 85%.
Key variables analyses
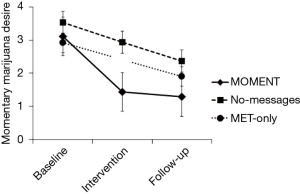
At follow-up vs. baseline, participants overall reported significantly higher PDA (Mdn =50% vs. 18.5%, Z = −4.01, P<0.0001) and lower POSIT scores (Mdn =1 vs. 4 all substances, Z = −2.73, P<0.0001; 1 vs. 3 marijuana, Z = −3.71, P<0.0001); these effects did not differ by arm. Momentary marijuana desire overall decreased from baseline to follow-up (change in desire rating 1.34, time main effect F=37.2, P<0.0005). There was a significant arm-by-phase interaction effect, with a greater decline in momentary marijuana desire with MOMENT, compared to MET-only (F=4.14, P=0.006; Figure 2). Marijuana use on momentary reports also decreased over the study (time main effect F=12.22, P<0.0005), with odds of use in the intervention and follow-up phases significantly lower than in the baseline phase [adjusted odds ratio (95% confidence interval): 0.46 (0.28–0.76) and 0.31 (0.19–0.51), respectively]. The arm-by-phase interaction was non-significant (P=0.71). However, in reports within 6 hours following an EMI-targeted context or behavior, post-hoc comparisons showed significant decreases from baseline to follow-up in MOMENT and No-messages, but not MET-only (adjusted use proportion difference −0.24, P=0.01 and −0.20, P=0.02 vs. −0.12, P=0.18; Figure 3).

Discussion
This pilot randomized trial demonstrated that the MOMENT intervention is feasible to deliver in primary care, acceptable, and potentially efficacious in reducing marijuana desire and use among frequently-using adolescent and young adults. Most participants who started treatment completed it and were retained at three months, similar to our single-group pilot (22) and other youth marijuana intervention studies (28,29). Most attrition occurred prior to any treatment and was higher among more-affected youth. These youth may have been deterred by study burden or had lower motivation to reduce or quit marijuana use (not determined because motivation was assessed at MET1). Insofar as change is a process and motivation can vary (30), youth in primary care may need repeated offers of treatment. Alternatively, as some participants’ comments suggested, considering or starting a study to reduce marijuana use may have motivated them to cut down or quit on their own. Momentary and daily response rates varied widely, but overall were similar to those reported in other EMA studies of marijuana use (31-33) and were improved from our single-group pilot (22). In general, youth highly rated the MET, enjoyed using the smartphone, and found study participation interesting, motivating, and helpful in reducing marijuana use.
Most participants were using marijuana at least daily and for many years, and had not received prior treatment. More than one-half had previously attempted to self-discontinue use. Brief interventions for primary care, where these youth may be engaged in care, are needed. Participants in all three conditions received MET and overall days of abstinence increased, and problems associated with substances generally and marijuana specifically decreased over the study. MOMENT’s brief manualized MET provides an evidence-based treatment approach while limiting the burden on primary care’s limited human and facility resources. MOMENT also offers an extended period of smartphone self-monitoring and messaging, capitalizing on youth engagement with mobile technology and need for treatment in real-life contexts. Compared to those with no mobile intervention (MET-only), youth receiving MOMENT had a greater decline in marijuana desire and, along with youth self-monitoring without messages, had a greater decline in use following EMI-targeted behaviors and contexts. Because this study’s focus was to assess feasibility in anticipation of a larger randomized trial (30), the primary purpose of these analyses was to evaluate the utility of the measures and develop analytic approaches. Although the findings show promise, further study in a larger sample will be important to determine MOMENT efficacy.
The study’s small sample size limited power to detect predictors of feasibility. Although recruitment occurred in five clinics, all were affiliated with a single academic children’s hospital, limiting generalizability to other types of primary care clinics serving adolescents and young adults. Recruitment and retention rates were modest and would likely improve with a more sophisticated app that allows participants use of a personal smartphone. Although overall acceptability was high, reducing the number of momentary prompts may minimize participant frustration, as well as increase responding (34). Future research can also address participants’ desire for varied message content, which may increase response and intervention effects. Perceived effectiveness was high immediately post-intervention, but declined substantially by 3-month follow-up, suggesting that boosting the intervention (i.e., another counseling session) or utilizing an adaptive design to provide more intensive intervention to non-responders warrants consideration in future studies.
Youth with chronic, frequent marijuana use are at the highest risk of neurocognitive and social harms (5-9). MOMENT offers a practical treatment option for primary care, where few alternatives exist outside of substance use treatment programs. The brief MET component of MOMENT is pragmatic and aligned with the principles of integrated behavioral health within the patient-centered medical home (35). The MOMENT EMI is both novel and appealing in its delivery of ecologically-relevant, low-cost intervention for marijuana use via youth-friendly technology. This study demonstrated that a randomized trial of MOMENT is feasible, and the intervention is acceptable and potentially helpful to the target population. Lessons learned should be applied in a larger randomized trial.
Acknowledgements
The authors acknowledge Bea Duvert, Meghann Soby, Allegra Spalding, Ken Winters, and Maya Mundkhur for their assistance in realizing this study.
Funding: This work supported by the National Institutes of Health [R34DA030535 to LA Shrier].
Footnote
Conflicts of Interest: Findings were presented in part at the Society for Adolescent Health and Medicine annual meeting, March 2016, Washington, D.C. and the Association of Medical Education and Research in Substance Abuse annual meeting, November 2016, Washington, D.C.
References
- Miech RA, Johnston LD, O'Malley PM, et al. Monitoring the Future national survey results on drug use, 1975-2015: Volume I, Secondary school students. Ann Arbor: Institute for Social Research, The University of Michigan, 2016.
- Substance Abuse and Mental Health Services Administration. Center for Behavioral Health Statistics and Quality, National Admissions to Substance Abuse Treatment Services. Rockville, MD: Substance Abuse and Mental Health Services Administration, 2012.
- Wu LT, Brady KT, Mannelli P, et al. Cannabis use disorders are comparatively prevalent among nonwhite racial/ethnic groups and adolescents: A national study. J Psychiatr Res 2014;50:26-35. [Crossref] [PubMed]
- Schulenberg JE, Johnston LD, O'Malley PM, et al. Monitoring the Future national survey results on drug use, 1975-2016: Volume II, College students and adults ages 19–55. Ann Arbor: Institute for Social Research, The University of Michigan, 2017.
- Gilman JM, Lee S, Kuster JK, et al. Variable activation in striatal subregions across components of a social influence task in young adult cannabis users. Brain Behav 2016;6. [Crossref] [PubMed]
- Gilman JM, Kuster JK, Lee S, et al. Cannabis use is quantitatively associated with nucleus accumbens and amygdala abnormalities in young adult recreational users. J Neurosci 2014;34:5529-38. [Crossref] [PubMed]
- Gruber SA, Dahlgren MK, Sagar KA, et al. Worth the wait: Effects of age of onset of marijuana use on white matter and impulsivity. Psychopharmacology (Berl.) 2014;231:1455-65. [Crossref] [PubMed]
- Meier MH, Caspi A, Ambler A, et al. Persistent cannabis users show neuropsychological decline from childhood to midlife. Proc Natl Acad Sci USA 2012;109:E2657-64. [Crossref] [PubMed]
- Rubino T, Zamberletti E, Parolaro D. Adolescent exposure to cannabis as a risk factor for psychiatric disorders. J Psychopharmacol 2012;26:177-88. [Crossref] [PubMed]
- Brook JS, Lee JY, Finch SJ, et al. Adult work commitment, financial stability, and social environment as related to trajectories of marijuana use beginning in adolescence. Subst Abus 2013;34:298-305. [Crossref] [PubMed]
- Silins E, Fergusson DM, Patton GC, et al. Adolescent substance use and educational attainment: An integrative data analysis comparing cannabis and alcohol from three Australasian cohorts. Drug Alcohol Depend 2015;156:90-6. [Crossref] [PubMed]
- Silins E, Swift W, Slade T, et al. A prospective study of the substance use and mental health outcomes of young adult former and current cannabis users. Drug Alcohol Rev 2017;36:618-25. [Crossref] [PubMed]
- Committee on Substance Abuse. Levy SJ, Kokotailo PK. Substance use screening, brief intervention, and referral to treatment for pediatricians. Pediatrics 2011;128:e1330-40. [Crossref] [PubMed]
- SIMmersion Immersive Simulations Training Center. Engaging Adolescent Patients About Marijuana Use. 2015 [cited 2015 December 21]. Available online: www.tinyurl.com/adolescentMI
- Hogue A, Henderson CE, Ozechowski TJ, et al. Evidence base on outpatient behavioral treatments for adolescent substance use: Updates and recommendations 2007-2013. J Clin Child Adolesc Psychol 2014;43:695-720. [Crossref] [PubMed]
- Li L, Zhu S, Tse N, et al. Effectiveness of motivational interviewing to reduce illicit drug use in adolescents: A systematic review and meta-analysis. Addiction 2016;111:795-805. [Crossref] [PubMed]
- Brown RA, Abrantes AM, Minami H, et al. Motivational interviewing to reduce substance use in adolescents with psychiatric comorbidity. J Subst Abuse Treat 2015;59:20-9. [Crossref] [PubMed]
- Dennis M, Godley SH, Diamond G, et al. The Cannabis Youth Treatment (CYT) Study: Main findings from two randomized trials. J Subst Abuse Treat 2004;27:197-213. [Crossref] [PubMed]
- Heron KE, Smyth JM. Ecological momentary interventions: Incorporating mobile technology into psychosocial and health behaviour treatments. Br J Health Psychol 2010;15:1-39. [Crossref] [PubMed]
- Bandura A. Social Foundations of Thought and Action: A Social Cognitive Theory. Englewood Cliffs, NJ: Prentice-Hall, 1986.
- Versluis A, Verkuil B, Spinhoven P, et al. Changing mental health and positive psychological well-being using Ecological Momentary Interventions: A systematic review and meta-analysis. J Med Internet Res 2016;18. [Crossref] [PubMed]
- Shrier LA, Rhoads A, Burke P, et al. Real-time, contextual intervention using mobile technology to reduce marijuana use among youth: A pilot study. Addict Behav 2014;39:173-80. [Crossref] [PubMed]
- Shrier LA, Rhoads AM, Fredette ME, et al. “Counselor in your pocket”: Youth and provider perspectives on a mobile motivational intervention for marijuana use. Subst Use Misuse 2013;49:134-44. [Crossref] [PubMed]
- Lane C, Huws-Thomas M, Hood K, et al. Measuring adaptations of motivational interviewing: The development and validation of the behavior change counseling index (BECCI). Patient Educ Couns 2005;56:166-73. [Crossref] [PubMed]
- Moyers TB, Rowell LN, Manuel JK, et al. The Motivational Interviewing Treatment Integrity Code (MITI 4): Rationale, preliminary reliability and validity. J Subst Abuse Treat 2016;65:36-42. [Crossref] [PubMed]
- Rahdert E. The Adolescent Assessment/Referral System Manual. DHHS Publication No (ADM)91-1735. U.S. Department of Health and Human Services, Alcohol, Drug Abuse, and Mental Health Administrations, 1991.
- Winters K, Henly G. Adolescent Diagnostic Interview (ADI) Manual. Los Angeles: Western Psychological Services, 1993.
- de Dios MA, Herman DS, Britton WB, et al. Motivational and mindfulness intervention for young adult female marijuana users. J Subst Abuse Treat 2012;42:56-64. [Crossref] [PubMed]
- Walker DD, Roffman RA, Stephens RS, et al. Motivational enhancement therapy for adolescent marijuana users: A preliminary randomized controlled trial. J Consult Clin Psychol 2006;74:628-32. [Crossref] [PubMed]
- Miller WR, Rollnick S. Motivational interviewing: Helping people change (3rd edition). Applications of motivational interviewing. New York: Guilford Press, 2013.
- Shrier LA, Walls CE, Kendall AD, et al. The context of desire to use marijuana: Momentary assessment of young people who frequently use marijuana. Psychol Addict Behav 2012;26:821-9. [Crossref] [PubMed]
- Black SK, de Moor C, Kendall AD, et al. Feasibility of momentary sampling assessment of cannabis use in adolescents and young adults. J Child Adolesc Subst Abuse 2014;23:177-84. [Crossref]
- Eldridge SM, Chan CL, Campbell MJ, et al. CONSORT 2010 statement: extension to randomised pilot and feasibility trials. BMJ 2016;355:i5239. [Crossref] [PubMed]
- Phillips KT, Phillips MM, Lalonde TL, et al. Marijuana use, craving, and academic motivation and performance among college students: An in-the-moment study. Addict Behav 2015;47:42-7. [Crossref] [PubMed]
- Benefits of NCQA Patient-Centered Medical Home Recognition. Washington, D.C.: National Committee for Quality Assurance, 2016.
Cite this article as: Shrier LA, Burke PJ, Kells M, Scherer EA, Sarda V, Jonestrask C, Xuan Z, Harris SK. Pilot randomized trial of MOMENT, a motivational counseling-plus-ecological momentary intervention to reduce marijuana use in youth. mHealth 2018;4:29.