Evaluation of the effectiveness of mobile diabetes management system with social networking and cognitive behavioural therapy (CBT) for T2D
Introduction
Long-term diabetes is suffered by almost 366 million persons around the world. This is an impressive number that points out the impact of this sickness worldwide and in the Kingdom of Saudi Arabia, where about 20% of the population suffers from diabetes (1). In this sense, statistics show that the region ranks in the seventh place among the nations of the world afflicted by this disease (1). Also, the estimations of the International Diabetes Federation (IDF), indicates that the highest rates of diabetes in 2011 are in five countries of this region (1). Projections of the IDF for the year 2030 show a similar behavior.
It is possible to argue that as a consequence of the economic progress of the region, the people of Saudi Arabia has assumed unhealthy eating practices that, combined with climatic influences, family history, lack of exercise, smoking habits, obesity, cultural aspects, social norms of behavior, and little information on health education have contributed to the high dominance of diabetes (2-4).
Regarding the mobile management systems of diabetes, research on this topic has increased globally in recent years (5-8). Also, social networks that have appeared recently represent alternatives for beneficiaries and patients to give and receive health care information (9). In this sense, some social sites such as PatientsLikeMe, CureTogether, MedHelp, mCare and others offer a variety of health services according to their respective intentions (10-14).
It is worth mentioning that most of these studies have been conducted in the United States of America, Europe and developed nations such as the United States of America and European countries. But, in the Gulf region, to date, no study has been carried out on the use of social networking, although this region is one of the world’s largest users of smart mobile phones and social sites like Facebook and Twitter (15). However, only three studies related to the use of mobile phones in Bahrain, short message service (SMS) in Iraq, and a mobile application in Qatar were detected in a review carried out recently by the authors on the use of the mobile diabetes management system and social networks in the Gulf region (16). In the research of Bahrain, it was found that the use of mobile phones among a group of diabetic patients improved results in hemoglobin A1C (17). Similarly, the investigation in Iraq indicated that the SMS upgraded outcomes in the education of diabetic patients (18). And, the mobile application designed in the Qatar study to aid patients to manage diabetes through glucose and diet control showed that the diabetic patient users were satisfied with the implemented mobile system (19). A recent review, confirmed that only a small number of investigations related to diabetes management with social networking systems have been carried out in the Middle East nations (20).
These scarce studies suggest the urgent need to conduct more research on this topic in the region, taking into account the challenges of the prevailing diabetes epidemic. Likewise, no research has been carried out with Saudi patients using social networks combined with the management of mobile diabetes.
Therefore, in this article, we investigate the clinical study of the Saudi Arabia Networking Aiding Diabetes (SANAD) system designed for Saudi type 2 diabetic patients. In this sense, the principal objective of this research is to examine the effect of the SANAD system on the improvement of glycaemic control, health awareness and self-efficacy among these patients. Regarding the organization of the article, Section II describes a scheme of the design and method employed in this work, Section III displays the results and Section IV shows the discussion and conclusions of this investigation.
Methods
SANAD system design
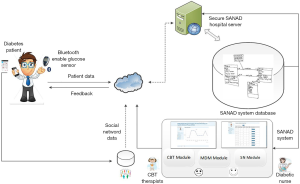
The SANAD system was designed to promote smart social behavioral change intervention and mobile management tailored to Saudi diabetic participants (21). The SANAD design is depicted in Figure 1. The system consists of three principal modules: a smart mobile diabetes management module (SMDMM), a social networking module (SNM), and a cognitive behavioral therapy module (CBT-M). The SMDMM was developed to collect blood glucose data wirelessly from Smartphone using Bluetooth technologies from the glucose sensors; the SNM acts as an improvement educational module for the SMDMM, and the CBT-M designed on the Smartphone platform is utilized for complementary behavioral change by patients who require cognitive behavior therapy (CBT) therapeutic intervention.
Study setting and participants
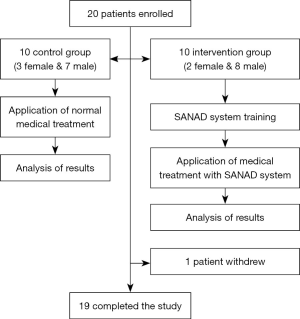
The total number of participants consisted of 20 type 2 diabetic patients: 15 men and 5 women. More males were recruited than females because the medical center was in the military area which has more men than women. All participants were from a clinic in Saudi Arabia-Damman. In Table 1 are shown the baseline demographic and clinical characteristics of the intervention and control groups’ participants. Also, Figure 2 depicts the study design. The eligibility criteria were: duration of type 2 diabetes >1 year, HbA1c <12% and age ≤50 years at the time of admission. And, the exclusion criteria were: pregnant women, and males and females with diabetes complications.
Full table
The research study was a randomized controlled trial (RCT) with an intervention group and a control group. The participants of the intervention group used the SANAD system and were trained to activate and operate the blood glucose sensors and transfer this information employing the SANAD application for smartphones. For this purpose, smartphones were given to the participants of the intervention group. Also, they learned how to use the smartphones, the social networking services, and how to execute the CBT operation. Group sessions of 30–45 minutes were conducted to inform participants about these operations. In contrast, the participants of the control group got normal medical treatment and management by the staff of the medical center.
The recruitment of the participants was done by the clinical personnel during an interview or using SMS messages. Before initiating the study, all participants signed a written informed consent. The ethical approval for this study was granted by the Institutional Review Board (IRB) with number IRB UGS 2015-03-001.
Procedure to evaluate glycemic control, DKT and DMSES
The primary outcome of this investigation was the glycemic control which is detected by the participants’ HbA1c levels. Secondary outcomes were health awareness assessed by the diabetes knowledge test (DKT), and behavioral change measured using the Diabetes Management Self-efficacy Scale (DMSES). A summary of the evaluation procedure is described below.
Evaluation of glycemic control
The Glycemic control was carried out by measuring the participants HbA1c levels. To do this procedure, blood samples from the participants were collected using an aqueous 3.0 µL finger stick from a blood sample collection kit. The evaluation of the percent of HbA1c among the participants was conducted by a multi technic sample screening using high-performance liquid chromatography-ion exchange to sense possible interferences, followed by high-performance liquid chromatography-bioassay analysis. The HbA1c level of the participants was measured at the time of enrollment and at 6-month intervals during the time of the study.
Evaluation of the DKT
The participant’s diabetes knowledge was evaluated using the DKT test (22). This test, developed by the Diabetes Education Study of Starr County, Texas, contains 24 items, and it is an abbreviated version of the 60-item survey from Garcia et al. (23). The reasonable answers to each question of the test are “yes”, “no”, and “I do not know”. If the answer is “yes” the participant receives 1 point if the answer is “no” or “I do not know” the participant gets 0 points. The test was performed at the beginning of the study and 6 months after the investigation began.
Evaluation of the DMSES
The Dutch/American DMSES evaluates the participant’s efficacy for engaging in 20 self-control activities of type 2 diabetes patients, such as daily exercise or a healthy eating plan when you are not at home (24). The level of efficacy expectation is rated according to a numeric scale of 1 to 10 points. The highest scores indicate higher levels of self-efficacy. The DMSES test was done at the beginning and every 6 months during the study.
Statistical analysis
The pre and post control intervention data were analyzed using the Statistical Package for Social Sciences (SPSS) version 21 (25). Statistical significance was defined as a probability P<0.05 (26). The power calculations were made with GPower 3.1.
The primary outcome measures were HbA1c (%), and the secondary outcomes were total knowledge of diabetes and average self-efficacy. Ten participants were evaluated in each group. The power calculation indicated that ten participants per group had 60% power (two faces, large effect size, P value =0.05) when the t-test of paired samples is used to compare the pre- and post-data at the baseline and after 6 months. The assumption of normality was met, with all asymmetry values close to 1 (27).
The main hypothesis test differences between the control and intervention groups over time were calculated using mixed analysis of variance (ANOVA) and paired t-tests (28). To assess the amount of change after 6 months from the baseline, the change scores for each outcome measure were calculated using the formula [time2 − time1]. In addition, the percentage of change for each outcome measure was calculated using the formula [(time2 − time1/time1) ×100]. Independent samples t-test and linear regression were then used to evaluate the impact of the intervention on the mean using the change scores for each measured result.
For the linear regression analyzes, the condition of the independent variable was (dummy coded as 0= control, 1= intervention group), and the three dependent variables were HbA1c (%), knowledge of diabetes and diabetes scores. Change of self-efficacy. Pearson correlations compared pre and post HbA1c scores (%), diabetes awareness and Self-Efficacy.
Results
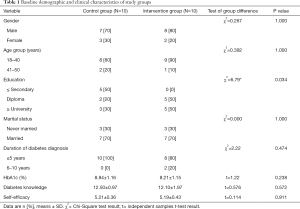
The baseline demographic and clinical characteristics of the intervention and control group’s participants are summarized in Table 1. The table shows the gender, age, education, marital status, duration of diabetes, the percentage of HbA1c, diabetes knowledge and self-efficacy of the participants of both groups. Likewise, the table presents the Chi-square and t-test results in terms of the statistical parameter χ2 and the t value. Also, the table presents the probability value P. According to the table, the participants were mainly men, the large majorities were aged 18–40 years, and were married, and the majority had a diagnosis of diabetes for five years or less. On average, participants scored 8–9 for HbA1c (%), 12–12.50 for diabetes knowledge, and 5 for self-efficacy scores.
Likewise, Table 2 displays the statistical paired t-test results of the pre and post values of HbA1c (%), diabetes knowledge and self-efficacy outcomes of the control and intervention groups. The table exhibits the mean, the standard deviation (SD), the mean change, the standard error of the mean (SEM), the t value, and the P value of the outcome measure with regard to the main hypothesis.

Full table
In a similar way, Table 3 illustrates the application of the statistical independent t-test and presents the HbA1c (%), diabetes knowledge and self-efficacy of the control and intervention groups. The table shows the mean change, the standard deviation (SD), the standard error of the mean (SEM), the t value, and the P value of the outcome measure.

Full table
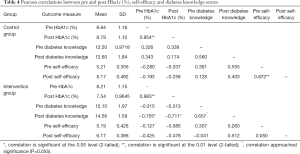
On the other hand, Table 4 shows the Pearson correlations for the pre and post values of HbA1c (%), diabetes knowledge and self-efficacy of the control and intervention groups. The table shows the mean and the SD significant at the 0.01, 0.05 and 0.055 levels.
Full table
Discussion
This feasibility study, which is the first evaluation of SANAD for diabetes type 2 patients in Saudi Arabia, provides evidence that SANAD has a positive impact on promoting knowledge of diabetes in individuals living with type 2 diabetes, and reflects the generally positive outcomes of reducing glycated hemoglobin control [HbA1c (%)], and increasing self-efficacy.
The results described in Table 1 indicates that the Chi-square and independent samples t-tests revealed that there were no significant differences between control and intervention group in demographic or clinical characteristics, except for education, which was higher in the intervention group (χ2(2)=6.79, P<0.05).
In relation with the level of diabetes knowledge, the findings presented in Tables 2-4 confirm that in our pre-post RCT the participants’ level of diabetes knowledge in the intervention group increased significantly after the intervention process. In this sense, Table 2 points out that the mean diabetes knowledge score prior to the intervention (baseline) was 12.11 (SD 2.09), which rose to 14.56 (SD 1.59) after the intervention. This increase [mean (SEM), 2.44 (0.530)] was shown to be significant using the paired-samples t-test (P=0.002). The independent samples t-test results depicted in Table 3 revealed that diabetes knowledge significantly increased in the intervention group by 2.44 points, and increased by 0.100 points in the control group, t(17) =−3.28, P<0.01. Also, Table 4 indicates that the Pearson correlation between diabetes knowledge scores before and after the SANAD intervention approached significance [r(9) =0.657, P=0.055]. Details about the nomenclature of t(17) and r(9) can be found in reference (29). Linear regression demonstrated that age, gender, and educational level were not related to increased diabetes knowledge in each study group (30). These results are consistent with the outcomes of Haddad et al., who found a mean pre-post increase of 1.29 points in diabetes knowledge using mobile phone text messages (31). Our study found an even larger mean increase of 2.44 points in knowledge (P<0.05). Further trials and clinical observation are required to determine whether this increase in diabetes knowledge is clinically sufficient.
Analyzing the glycemic control results described in Table 2, we observe that the baseline HbA1c (%) was 8.14 (SD 1.20) and decreased to 7.54 (SD 0.96) after the SANAD intervention [mean (SEM) decrease 0.600 (0.102)]. Similarly, the paired-samples t-test showed this change to be significant (P=0.000). In Table 3, the independent samples t-test compared mean change in HbA1c (%) scores between the control and intervention group, and revealed that HbA1c (%) decreased by 0.600 points in the intervention group, but decreased by only 0.050 points in the control group, and this difference was significant, t(17) =3.63, P<0.01. The Pearson correlation depicted in Table 4 suggests that the baseline and 6-month HbA1c values were positively and strongly correlated for both intervention and control group [r(9) =0.983, P<0.01; and r(10) =0.954, P<0.01, respectively]. Linear regression analyzed each study group separately, and revealed that age significantly predicted change in HbA1c (%) in the intervention group, with older age (i.e., 41–50 years) associated with higher HbA1c (%) at 6 months, as compared with baseline values (β=0.865, t=3.67, P<0.05) (26). Gender and educational level did not predict the change in HbA1c (%). In the control group, education level predicted the change in HbA1c (%) (β=−0.714, t=4.94, P<0.05), with lower education associated with higher HbA1c (%) at 6 months, as compared with baseline values. Briefly, the mean glycemic control in the intervention group achieved a significant mean decrease of 0.600 points from baseline levels (P<0.01), whereas the mean HbA1c concentration in the control group decreased only 0.05 points from baseline levels. This finding suggests that the SANAD system is comparably effective with the SMS text messaging for glycemic control investigated by Haddad et al. (31) who found a significant change in HbA1c levels (P<0.001) with a mean decrease of 8.6 (%) in type 2 diabetes patients; and it is as effective as the similar Sweet Talk device of the Franklin et al. study (32) which decreased intervention patients HbA1c concentration by 9.2 points (P<0.001). It is convenient to mention that the Sweet Talk system is a text message system accompanied with psychological strategies capable of motivating the behavior of diabetic patients.
With respect to the self-efficacy outcomes of the control and intervention groups, Table 2 indicates that the mean self-efficacy score prior to the intervention was 5.17 (SD 0.45), which then rose to 6.17 (SD 0.39) afterward. This increase [mean (SEM), 0.944 (0.192)] was shown to be significant using the paired-samples t-test (P=0.001). Table 3 presents that the independent samples t-test points out that self-efficacy significantly increased in the intervention group by 0.994 points, but decreased in the control group by 0.035 points, t(17) =−4.94, P<0.001). According to the Pearson correlation presented in Table 4, there was no correlation between self-efficacy scores before and after the SANAD intervention [r(9) =0.050, P=0.899]. Linear regression demonstrated that age, gender, and educational level were not related to increased self-efficacy in each study group (30). In this sense, self-efficacy improved significantly in our SANAD intervention patients by 0.994 points from baseline scores (P<0.001). This result is comparable to the Sweet Talk associated enhancement in self-efficacy found by Franklin et al., who detected that it improved by 62.5% (P<0.01) in their intervention group (32).
In summary, the SANAD application combines the advantages for improving mean glycemic control and diabetes knowledge as found by Haddad et al. using SMS; and shares the improvements in self-efficacy detected by Franklin et al., using Sweet Talk, a mobile phone text messaging support network. In addition, SANAD features include videos and communication between patients and physicians, making it a more efficient system for improving all three target outcomes. A previous usability study found that the SANAD system is well received by diabetes patients in Saudi Arabia, but as a first development, it obviously requires some upgrade to increase satisfaction (33).
This study has revealed notable strengths of the SANAD system. For example, it is the first mobile diabetes management system to be developed and tailored for Saudi diabetes type 2 adult patients, to enhance their diabetes type 2 management, including improving glycemic control, diabetes knowledge, and self-efficacy for behavioral change, based on CBT. Also, it was easy to recruit type 2 diabetes patients from one healthcare clinic in Dammam, Saudi Arabia. Likewise, this study is the first randomized clinical controlled trial in the Gulf area, particularly in Saudi Arabia to evaluate the effectiveness of SANAD for the type 2 adult diabetes. A recent usability study (33) provides evidence that the SANAD system is easy to use.
Some of the limitations of this study that may affect internal and external validity include the small sample size (n=20), which was due to limited funding. This limitation affects the statistical power of this research. An additional limitation was that one participant left the intervention group because he was transferred to another clinical. Also, recruiting from one small healthcare clinic in one region (Dammam) is a limitation that reduced the generalization of the results; however, this is an exploratory pilot RCT; therefore, it was not intended to generalize the results of this population. Another limitation is the fact that we haven’t adjusted the findings of this research for the difference at the baseline education levels of the participants of the intervention and control groups. This situation may have facilitated their information processing and retention of diabetes information, over and above the effects of the SANAD intervention. Similarly, the issue of using self-report tools (e.g., to measure self-efficacy) may be associated with common method bias (e.g., mood bias, socially desirable responding). In any case, it is possible that intervention participants may have felt grateful for receiving a top branded smartphone and felt obligated to report better self-efficacy. However, the finding that change in self-efficacy and glycemic control was uncorrelated among intervention participants adds some validity to the limitation of self-report findings.
It is convenient to mention that ethical approval from Saudi government sector was difficult to obtain in terms of time (a very slow process). Also, it was also difficult to gain ethical approval from Kingston University London for this study. Finally, the ethical approval for this study was granted by the Institutional Review Board (IRB) with number IRB UGS 2015-03-203.
In conclusion, we present a clinical study of SANAD system among Saudi type 2 diabetes participants. The key outcomes of the SANAD clinical study determine that SANAD has a positive impact on promoting knowledge of diabetes in individuals living with type 2 diabetes, and reflects the generally positive outcomes of reducing glycated hemoglobin control [HbA1c (%)], and increasing self-efficacy.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: Before initiating the study, all participants signed a written informed consent. The ethical approval for this study was granted by the Institutional Review Board (IRB) with number IRB UGS 2015-03-001.
References
- 5th Edition of the Diabetes Atlas released on World Diabetes Day | International Diabetes Federation [Internet]. [cited 2015 Jan 01]. Available online: http://diabetesatlas.org/resources/2017-atlas.html
- Al-Hazzaa HM. Prevalence of physical inactivity in Saudi Arabia: a brief review. East Mediterr Health J 2004;10:663-70. [PubMed]
- Elhadd TA, Al-Amoudi AA, Alzahrani AS. Epidemiology, clinical and complications profile of diabetes in Saudi Arabia: a review. Ann Saudi Med 2007;27:241-50. [Crossref] [PubMed]
- Chopra M., Galbraith S, Darnton-Hill I. A global response to a global problem: the epidemic of overnutrition. Bull World Health Organ 2002;80:952-8. [PubMed]
- Istepanian RS, Zitouni K, D., Harry D, et al. Evaluation of a mobile phone telemonitoring system for glycaemic control in patients with diabetes. J Telemed Telecare 2009;15:125-8. [Crossref] [PubMed]
- Earle KA, Istepanian RS, Zitouni K, et al. Mobile telemonitoring for achieving tighter targets of blood pressure control in patients with complicated diabetes: a pilot study. Diabetes Technol Ther 2010;12:575-9. [Crossref] [PubMed]
- Istepanian RS, Woodward B. m-Health: Fundamentals and Applications. 1st ed. New Jersey, NJ: Wiley-IEE Press, 2016.
- Liang X, Wang Q, Yang X, et al. Effect of mobile phone intervention for diabetes on glycaemic control: a meta‐analysis. Diabet Med 2011;28:455-63. [Crossref] [PubMed]
- Pearson JF, Brownstein CA, Brownstein JS. Potential for electronic health records and online social networking to redefine medical research. Clin Chem 2011;57:196-204. [Crossref] [PubMed]
- Patientslikeme. 2013 [Internet]. [cited 2015 Jan 01] Available online: http://www.patientslikeme.com/
- Curetogether [Internet]. [cited 2015 Jan 01] Available online: http://curetogether.com/
- MedHelp [Internet]. [cited 2015 Jan 01] Available online: http://www.medhelp.org/
- Yu WD, Siddiqui A. Towards a wireless mobile social network system design in healthcare. Multimedia and Ubiquitous Engineering 2009. MUE’09. Third International Conference, 2009:429-36.
- Swan M. Emerging patient-driven healthcare models: an examination of health social networks, consumer personalized medicine, and quantified self-tracking. Int J Environ Res Public Health 2009;6:492-525. [Crossref] [PubMed]
- Saudi has the world’s largest number of mobile phone users: U.N. report [Internet]. [cited 2018 Apr 10] Available online: https://www.statista.com/statistics/558821/number-of-mobile-internet-user-in-saudi-arabia/
- Alanzi T, Istepanian R, Philip N. Mobile Diabetes management system embedding social networking in the Gulf countries: the current status and potential impact. Diabetes Technol Ther 2014.149.
- Hussein WI, Hasan K, Jaradat AA. Effectiveness of mobile phone short message service on diabetes mellitus management; the SMS-DM study. Diabetes Res Clin Pract 2011;94:e24-e26. [Crossref] [PubMed]
- Mulvaney D, Woodward B, Datta S, et al. Development of m-health monitoring systems in India and Iraq. Engineering in Medicine and Biology Society (EMBC), 2012 Annual International Conference of the IEEE, 2012:288-91.
- Alhazbi S, Alkhateeb M, Abdi A, et al. Mobile application for diabetes control in Qatar. Computing technology and information management (ICCM). 8th International Conference, 2012.
- Alanzi T. Role of social media in diabetes management in the Middle East Region: systematic review. J Med Internet Res 2018;20. [Crossref] [PubMed]
- Alanzi T, Istepanian R, Philip N. Design and Usability Evaluation of Social Mobile Diabetes Management System in the Gulf Region. JMIR Res Protoc 2016;5. [Crossref] [PubMed]
- Garratt AM, Schmidt L, Fitzpatrick R. Patient-assessed health outcome measures for diabetes: A structured review. Diabet Med 2002;19:1-11. [Crossref] [PubMed]
- Garcia AA, Villagomez ET, Brown SA, et al. The Starr County Diabetes Education Study: Development of the Spanish-language diabetes knowledge questionnaire. Diabetes Care 2001;24:16-21. [Crossref] [PubMed]
- Bijl JV, Poelgeest-Eeltink AV, Shortridge-Baggett L. The psychometric properties of the diabetes management self-efficacy scale for patients with type 2 diabetes mellitus. J Adv Nurs 1999;30:352-9. [Crossref] [PubMed]
- IBM SPSS statistics 21.0 Available for Download-Unites States [Internet]. [cited 2018 April 10]. Available online: https://www-01.ibm.com/support/docview.wss?uid=swg21608060
- Zar JH. Biostatistical Analysis. Pearson Education India, 2010:663.
- Field AP. Discovering Statistics Using SPSS. London: SAGE Publications, 2009;58:821.
- Discovering statistics. Mixed factorial Anova [Internet]. [cited 2018 April 10]. Available online: https://www.discoveringstatistics.com/repository/mixed.pdf
- Methods manual: t-test, hand calculation- UC Davis, Psychology [Internet]. [cited 2018 April 10]. Available online: http://psc.dss.ucdavis.edu/sommerb/sommerdemo/stat_inf/tutorials/ttesthand.htm
- Regression methods [Internet]. [cited 2018 April 10]. Available online: https://onlinecourses.science.psu.edu/stat501/node/250
- Haddad NS, Istepanian R, Philip N, et al. A Feasibility Study of Mobile Phone Text Messaging to Support Education and Management of Type 2 Diabetes in Iraq. Diabetes Technol Ther 2014;16:454-9. [Crossref] [PubMed]
- Franklin VL, Greene A, Waller A, et al. Patients’ engagement With ‘Sweet Talk’-a text messaging support system for young people with diabetes. J Med Internet Res 2008;10. [Crossref] [PubMed]
- Alanzi T, Istepanian R, Philip N. Usability study of mobile social networking system among Saudi Type 2 diabetes patients (SANAD). 2nd Middle East Conference on Biomedical Engineering 2014:297-300.
Cite this article as: Alanzi T, Alanazi NR, Istepanian R, Philip N. Evaluation of the effectiveness of mobile diabetes management system with social networking and cognitive behavioural therapy (CBT) for T2D. mHealth 2018;4:35.