Advancing evidence-based digital health through an innovative research environment: an academic-industry collaboration case report
Introduction
The ubiquitous use of technology galvanizes both academic researchers and entrepreneurs in population health. Intersecting in the field of digital health, academic-industry collaborations (AICs) play a critical role in advancing science into real world application (1,2). Examples of successful AICs include traditional biotechnology (biotech) innovation models, academic entrepreneurship efforts, and government-sponsored technology partnership programs (3-8). Such partnerships seek to balance both scientific rigor and commercial viability, yielding sustainable evidence-based digital health products (9). Collaboration models vary but often exclude an emphasis on iterative rapid research, an approach the pace of technology demands (3-8).
Various barriers limit the ability to establish digital health AICs that promote responsive evaluation. While not explicitly unique to the field of digital health, scarce research dollars, limited recruitment pools, and strict institutional infrastructure affect collaboration success (10-13). Additionally, conflicting priorities and timelines exacerbate the challenges between academic and industry partners (12,14-16).
University-integrated health systems offer a fruitful environment to foster AICs, thus advancing innovation and science in digital health (10,17-19). Academic Health Centers (AHCs) encompassing health profession schools (e.g., medicine, public health, pharmacy, and nursing) and an affiliated health system offer the access and support required for translating validated innovations into practice (20). Proposed as “catalysts” to digital health success (10), AHCs leverage interdisciplinary expertise, patient data, and clinical trial infrastructure to advance novel digital health products. AHC-based AICs (AHC-AICs) offer promise in tackling the barriers described, yielding evidence-based technologies without thwarting innovation (1,14,21-23).
At least 70 university digital health centers exist within AHCs in the United States (24), but digital health AICs in this context are unexplored in the literature (20,23). The purpose of this paper describes a nascent academic-industry partnership designed to rapidly implement and test digital health products. This report specifically examines the research partnership between multiple industry technology partners and an AHC that includes: the UCHealth health delivery system, the CARE Innovation Center (CARE), CU Innovations (technology transfer office), and the mHealth Impact Lab. The case report synthesizes findings based on initiated (n=9) and completed (n=2) digital health projects conducted through the CARE and supported by the mHealth Impact Lab in 2018. Projects spanned a variety of digital modalities including but not limited to sensors, wearables, and mobile applications. The setting of all proposed research was within the UCHealth system and included 4 different industry technology partners. Based on qualitative feedback obtained by AHC-AIC stakeholders, this report presents lessons learned and future considerations with digital health AHC-AICs in practice.
Case presentation
Key partners
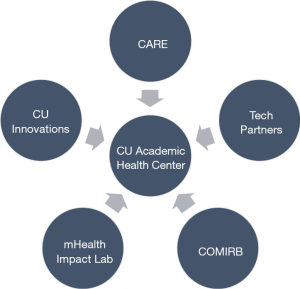
As seen in Figure 1, the AHC partnership includes five key partners: (I) a university health system, (II) innovation technology transfer office, (III) industry-AHC convener, (IV) research support collaborators, and (V) multiple technology industry partners.
The University of Colorado Anschutz Medical Campus (AMC) is an academic health center that includes multiple health profession schools, centers, and institutes. AMC houses two university teaching hospitals, treating roughly 2 million patients per year (25). The UCHealth System encompasses 2,000 providers, 150 clinics, and 10 hospitals in Colorado with affiliated hospitals in Wyoming and Nebraska.
CARE situates within UCHealth, and has financial support from CU Innovations, the technology transfer office of the University of Colorado. Both CU Innovations and CARE convene industry partners, both startup and established, to implement and test emerging health technologies within the health system infrastructure.
Located at AMC, the mHealth Impact Lab is an electronic research organization (eRO) that operates through the Colorado School of Public Health. The mHealth Impact Lab works with academic and industry partners to create and curate high quality technologies aimed to improve health promotion, disease prevention, and health care. For this specific partnership, the mHealth Impact Lab assists with proposal consulting and research implementation support, and serves as the liaison between CARE and the Colorado Multiple Institutional Review Board (COMIRB).
Lastly, the AHC-AIC facilitates projects with diverse types of technology enterprises from small to large industry partners. These include: smaller digital health startups looking to pilot test or accelerate products through clinical validation; enterprises with advanced research and data science expertise, seeking larger scale partnership; technology developers, seeking to facilitate academic ideas through software programming; and larger established technology companies to clinically validate suites of digital health solutions through established AHC infrastructure.
Innovation workflow
While academic innovation occurs in a variety of ways across US institutions (22), CU Innovations and the CARE Innovation Center prioritize clinical validation of digital health tools within the AHC. This approach expands on the traditional innovation tech transfer process (26) to include research and evaluation in addition to education, vetting, incubation, acceleration, and launch of a particular digital health product to market.
When a potential technology industry partner emerges, CU Innovations and CARE stakeholders convene the Innovation Council (iCouncil) to assess the AHC-AIC opportunity. The iCouncil consists of multi-disciplinary stakeholders and subject matter experts for vetting. This innovation workflow reflects the importance of healthcare as a complex adaptive system (27) when testing digital and mHealth tools in practice. The clinical validation process is evolving depending on the context of the industry technology partner.
Implementation of partnership and clinical validation process
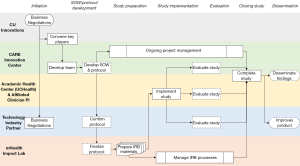
The summary for the clinical validation process and partnership implementation can be found in Figure 2. Detailed steps throughout the clinical validation process are found in Table 1.
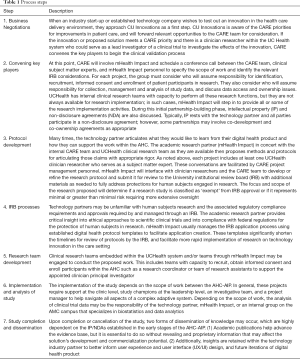
Full table
Case assertions
The AHC sees opportunity in improving patient care and saving costs, but waiting for technologies to become commercially available can be inefficient. Additionally, commercially available options have usually come to market without a rigorous analysis of their clinical validity, limiting their appeal to clinicians. Technology companies have products but lack large sample populations for implementation and testing, especially at early stages. Research teams have expertise in the design and ethics of conducting clinical research, but do not always have access to patient populations or emergent technologies. This partnership integrates these five partners to maximize the rapid, rigorous investigation of technologies that could potentially benefit patients and improve both the quality and cost of care.
Lessons learned
Leverage strengths of each partner
The literature proposes the potential for AHCs to leverage academic and industry expertise to advance digital health, and that potential was realized in this AHC-AIC (14,21). UCHealth offered the partnership a variety of strengths to advance digital and mobile health research. Such strengths included the available infrastructure and access to patient populations to include as research participants, clinician researchers who wanted to improve patient care, and administrators who were motivated to cut costs in care delivery without compromising care (10,18,28). Research partners like the mHealth Impact Lab offered skills in conducting rigorous human subjects research related to technology with a strong understanding of the field of digital health. This expertise in the design and delivery of clinical research, both in the design and delivery of research, facilitated the work in a productive ethical way. Industry technology partners thrive in ‘fail fast’ mentality and business processes (29,30). Such culture expedited study timelines and offered momentum for academic partners. Depending on the entry point of industry, these partners had capacity to quickly iterate on new technology innovations that may have the potential to impact health, care delivery, and health outcomes. A focus on commercialization demands designing for dissemination principles, an area where many academic interventionists fall short.
Required skillsets for AHC-AIC in practice
As the partnership evolved, specific skills were identified as needed and distributed across partners to alleviate various barriers. Maintaining the validity of a study can be challenging, thus clinical buy-in remains critical for study execution. A key facilitator for this rapid research was strong clinical support and clinical leadership to champion the work within the academic medical center (10,31). Such relationships executed the trials in a way that maximized access to patient populations and improved study implementation. Additionally, strong scientific investigative skills were needed to rigorously validate and project management skills to maintain strict timelines that navigate all aspects of complex adaptive systems (32,33).
The critical role of the IRB
IRBs must operate independently and according to strict federal regulations, thus they may not always have capacity to respond quickly to review innovation protocols on rapid, industry-familiar timelines. Under this initiative, the CARE developed agreements with COMIRB to dedicate resources to facilitate priority review of CARE protocols submitted by the mHealth Impact Lab. Additional pre-review and communication processes were established to ensure minimal required changes and timely notification of review-related events. The team also developed a strong understanding of the breadth of technologies cycling through the system and provided substantial supporting documentation for each, helping the IRB make determinations quickly.
Partnerships alone are not enough to overcome barriers
Balancing scientific rigor while considering commercial viability proves challenging (12,14-16). After a significant learning curve, the partners established role clarity and timeline expectations. This helped maneuver through institutional bureaucracy that rapidly worked with COMIRB to submit and implement studies. The scope of work for each project helped refine this process, however pragmatic barriers persisted that were primarily out of the partnership’s control. Such examples included technology device changes during studies, study site clinic complexity and competing projects underway in similar patient populations, limited bandwidth for IRB reviewers, and other regulatory factors that resulted in review timeframes inconsistent with industry partners’ more rapid development timeline-based expectations While this avenue of collaboration shows potential, establishing partnerships alone is not enough to overcome all logistical and ethical barriers among institutions; clear and ongoing communication is essential.
Recommendations
Cross-disciplinary flexibility
The partnerships thus far have worked with various technology products, addressed multiple health topics, and targeted diverse patient populations. This diversity required flexibility where protocols are similar among projects but with substantive changes in detail depending on terms agreed in a memorandum of understanding (MOU). Adapting to each study’s particular needs demanded an iterative process to ensure research objectives were pursued in a timely and ethical way that is appropriate to answer research questions with minimal burden placed on patients and providers.
Established relationship with IRB and research administration
The mHealth Impact Lab maintained regular, ongoing communication with COMIRB and with clinical research administration leadership at UCHealth. A team continually reviewed and discussed process improvement initiatives to ensure that protocol modifications related to changes with technology device, patient recruitment, or adaptive trial designs were minimized. This positive relationship allowed the partnership to test digital health solutions in a structured and rapid way.
Improving IRB understanding by industry
Upon initial partnership, collaborators identified the lack of awareness of IRB processes among technology industry partners as a barrier to successful implementation. While technology partners were collecting data and quality improvement information regularly for their products, these enterprises were unaware of federal and state regulations related to human subjects research and the ethical approaches of conducting formal scientific studies. Without this understanding, there was limited appreciation for the role of the IRB, the importance of IRB review, and the associated elongation of planning timelines in order to obtain IRB approval. For this AHC-AIC, the mHealth Impact Lab acted as an expert resource and IRB liaison, providing raised awareness of IRB processes and helping to manage timeline expectations and improve project planning. This allowed the partnership to improve project planning and execution without requiring each industry partner to develop a robust in-house understanding of the regulations, which would be impractical and inefficient.
Empathize with and explicitly address diverse values and priorities
Partners held goals in common, but also had conflicting value propositions due to their diverse business needs. Understanding and balancing these differing priorities was key to content partners and to successful partnership. Identifying disparate priorities early helped with a more rapid implementation of projects. This partnership welcomed conversations around intellectual property in the initial stages, allowing for explicit scopes of work and MOUs. Revisiting these discussions regularly was critical for the success of all partners: scholarship helped advance the evidence base, but it is important to do that without revealing confidential information that affects the industry partner’s development and commercialization potential.
Discussion
Researchers, clinicians, and entrepreneurs do not typically collaborate in the design, development, evaluation and dissemination of mHealth solutions, and are often found to be operating in parallel rather than collaboratively (14). Products such as wearables, mobile applications, telemedicine technologies, web-based platforms, and health information websites are developed at a rapid pace within the technology industry, but are not routinely evaluated using scientific methods in partnership with academic researchers (34-36).
The assertions of this case report highlight an approach for AICs within AHCs to aid an innovation environment that promotes advancing evidence-based technologies intended to improve health outcomes. While there are many strengths and limitations to consider with these partnerships, the focus of this case report highlights rapid and responsive research within the AHC. An AHC-AIC may help gain access to populations, build capacity to research digital health products, rapidly, ensure clinical expertise is engaged, promote ethical human subjects handling, and access innovative products from the technology industry (10,14,31). Strengths of AICs present the potential to expand funding possibilities, foster rapid development and research cycles, and design for dissemination, thus yielding sustainable products proven to enhance health outcomes.
This case report offers a high-level snapshot to describe an AHC-AIC. These partnerships are dynamic in nature and evolve by project and collaboration thus challenging generalization. This limitation offers promise in future research in learning from AICs in digital health. Many AICs exist (3-8); while this is just one model, additional research is needed to develop a formal consensus. This case is one that offers a model for rapid, rigorous research, allowing technology companies a platform to test and iterate their minimum viable products to generate both a greater value proposition and offer hospital systems ways to explore potential improvements in patient care. Currently, there lacks consensus of an AIC structure for ethical, robust research that can be responsive to the fast paced, quickly evolving mHealth technology environment. A formal review is needed to further understand academic and industry partnerships, models of collaboration, and best practices to advance evidence-based digital and mHealth solutions.
Conclusions
This case report contributes to the scarce body of literature on how academic industry collaborations in AHCs facilitate relevant digital health research. This report illustrates one of many ways AICs occur, bringing health systems, academia, and technology industries together to advance high quality digital and mobile health solutions. AHCs provide promise for rapidly testing technologies in real world settings, offering an intersection to implement AICs in a way to focus on rapid clinical validation. While more understanding is needed of best practices, AHCs offer a promising environment that considers strengths of each disciplines and intersects required skills and resources to advance the work. As the opportunity for mHealth and digital technologies increases, the demand for rigorously evaluated solutions produced by academic and industry is inevitable. Productive collaboration between fields is required to ensure digital health products are evidence-based, theory informed, and reach communities to positively influence health outcomes.
Acknowledgments
The authors wish to thank the University of Colorado Anschutz Medical Campus and CU Innovations for their contributions to the academic health center partnership, specifically Kimberly A. Muller, Esq. (CU Innovations Managing Director).
Funding: Dr. Portz’s is funded by a career development award from the National Institution on Aging (K76AG059934).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Fort DG, Herr TM, Shaw PL, et al. Mapping the evolving definitions of translational research. J Clin Transl Sci 2017;1:60-6. [Crossref] [PubMed]
- Brownson RC, Colditz GA, Proctor EK. Dissemination and implementation research in health: translating science to practice. Oxford University Press; 2017.
- Gnyawali DR, Park BJ. Co-opetition and Technological Innovation in Small and Medium-Sized Enterprises: A Multilevel Conceptual Model. J Small Bus Manag 2009;47:308-30. [Crossref]
- Iyawa GE, Herselman M, Botha A. Digital Health Innovation Ecosystems: From Systematic Literature Review to Conceptual Framework. Procedia Comput Sci 2016;100:244-52. [Crossref]
- Mention A-L. Co-operation and co-opetition as open innovation practices in the service sector: Which influence on innovation novelty? Technovation 2011;31:44-53. [Crossref]
- Ritala P, Sainio L-M. Coopetition for radical innovation: technology, market and business-model perspectives. Technol Anal Strateg Manag 2014;26:155-69. [Crossref]
- Flood J, Minkler M, Hennessey Lavery S, et al. The Collective Impact Model and Its Potential for Health Promotion: Overview and Case Study of a Healthy Retail Initiative in San Francisco. Health Educ Behav 2015;42:654-68. [Crossref] [PubMed]
- Etzkowitz H, Zhou C. The triple helix: University–industry–government innovation and entrepreneurship. Routledge; 2017.
- Ankrah S, Al-Tabbaa O. Universities–industry collaboration. A systematic review. Scand J Manag 2015;31:387-408. [Crossref]
- Depasse JW, Chen CE, Sawyer A, et al. Academic Medical Centers as digital health catalysts. Healthcare 2014;2:173-6. [Crossref] [PubMed]
- Patrick K, Hekler EB, Estrin D, et al. The Pace of Technologic Change: Implications for Digital Health Behavior Intervention Research. Am J Prev Med 2016;51:816-24. [Crossref] [PubMed]
- Yardley L, Choudhury T, Patrick K, et al. Current issues and future directions for research into digital behavior change interventions. Am J Prev Med 2016;51:814-5. [Crossref] [PubMed]
- Grundy QH, Wang Z, Bero LA. Challenges in Assessing Mobile Health App Quality: A Systematic Review of Prevalent and Innovative Methods: A Systematic Review of Prevalent and Innovative Methods. Am J Prev Med 2016;51:1051-9. [Crossref] [PubMed]
- Hingle M, Patrick H, Sacher PM, et al. The Intersection of Behavioral Science and Digital Health: The Case for Academic–Industry Partnerships. Health Educ Behav 2019;46:5-9. [Crossref] [PubMed]
- Moore C, Werner L, BenDor AP, et al. Accelerating Harmonization in Digital Health. World Health Popul 2017;17:43-54. [Crossref] [PubMed]
- Lim SY, Anderson EG, editors. Institutional Barriers Against Innovation Diffusion: From the Perspective of Digital Health Startups. 2016 49th Hawaii International Conference on System Sciences (HICSS); 2016: IEEE.
- Kohn LT, Institute of Medicine. Committee on the Roles of Academic Health Centers in the 21st C. Academic health centers: leading change in the 21st century. Washington, DC: Washington, National Academies Press; 2004.
- Toner M, Tompkins RG. Invention, innovation, entrepreneurship in academic medical centers. Surgery 2008;143:168-71. [Crossref] [PubMed]
- Ostrovsky A, Barnett M, editors. Accelerating change: fostering innovation in healthcare delivery at academic medical centers. Healthcare; 2014: Elsevier.
- Tseng J, Samagh S, Fraser D, et al., editors. Catalyzing healthcare transformation with digital health: Performance indicators and lessons learned from a Digital Health Innovation Group. Healthcare; 2018: Elsevier.
- Abroms LC, Allegrante JP, Auld ME, et al. Toward a Common Agenda for the Public and Private Sectors to Advance Digital Health Communication. Am J Public Health 2019;109:221-3. [Crossref] [PubMed]
- Ellner AL, Stout S, Sullivan EE, et al. Health systems innovation at academic health centers: leading in a new era of health care delivery. Acad Med 2015;90:872-80. [Crossref] [PubMed]
- Sharma A, Harrington RA, McClellan MB, et al. Using digital health technology to better generate evidence and deliver evidence-based care. J Am Coll Cardiol 2018;71:2680-90. [Crossref] [PubMed]
- Hostetter M, Klein S, McCarthy D, et al. Findings from a survey of health care delivery innovation centers. The Commonwealth Fund [Internet] 2015. Available online: https://www.commonwealthfund.org/publications/publication/2015/apr/findings-survey-health-care-delivery-innovation-centers
- University of Colorado. CU Facts and Figures 2017-2018. Accessed on September 2019. Available online: https://www.cu.edu/cu-facts-and-figures
- Mowery DC, Nelson RR, Sampat BN, et al. Ivory tower and industrial innovation: University-industry technology transfer before and after the Bayh-Dole Act. Stanford University Press; 2015.
- Lipsitz LA. Understanding health care as a complex system: the foundation for unintended consequences. JAMA 2012;308:243-4. [Crossref] [PubMed]
- Dzau VJ, Ackerly DC, Sutton-Wallace P, et al. The role of academic health science systems in the transformation of medicine. Lancet 2010;375:949-53. [Crossref] [PubMed]
- Khanna R, Guler I, Nerkar A. Fail often, fail big, and fail fast? Learning from small failures and R&D performance in the pharmaceutical industry. Acad Manag J 2016;59:436-59. [Crossref]
- Schaller RRJIs. Moore's law: past, present and future. IEEE Spectrum 1997;34:52-9. [Crossref]
- Mann DM, Chokshi SK, Lebwohl R, et al. Building digital innovation capacity at a large academic medical center. NPJ Digit Med 2019;2:13. [Crossref] [PubMed]
- Begun JW, Zimmerman B, Dooley K. Health care organizations as complex adaptive systems. In: Mick SM, Wyttenbach M, editors. Advances in Health Care Organization Theory. San Francisco: Jossey-Bass, 2003;253-88.
- Rouse WB. Health care as a complex adaptive system: implications for design and management. Bridge (Wash D C) 2008;38:17.
- Ioannidis JPA. Stealth Research: Is Biomedical Innovation Happening Outside the Peer-Reviewed Literature? JAMA 2015;313:663-4. [Crossref] [PubMed]
- Bull S. Beyond acceptability and feasibility: moving mHealth into impact. mHealth 2016;2:45. [Crossref] [PubMed]
- Informatics IIfH. Patient adoption of mHealth: use, evidence and remaining barriers to mainstream acceptance. IMS Institute for Healthcare Informatics Parsipanny, NJ; 2015.
Cite this article as: Ford KL, Moore SL, Zhou S, Gore MO, Portz J, Zhang X, Zane R, Wiler J, Bull S. Advancing evidence-based digital health through an innovative research environment: an academic-industry collaboration case report. mHealth 2019;5:37.