
Can mHealth and eHealth improve management of diabetes and hypertension in a hard-to-reach population? —lessons learned from a process evaluation of digital health to support a peer educator model in Cambodia using the RE-AIM framework
Introduction
The burden of non-communicable disease (NCD) is on the rise across the world, with a disproportionate impact felt by people living in low and middle-income countries (LMICs). Four out of five NCD deaths (80%) occur in LMICs (1,2), with NCDs rising faster than the decline of infectious diseases (3) and affecting far younger populations and with much worse outcomes than in high income countries (HIC) (4).
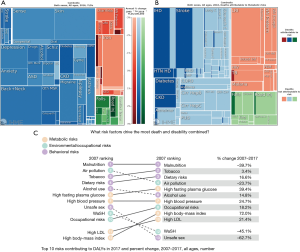
In Cambodia, where the life expectancy has increased over 15 years since 1990 due to this epidemiological transition, so too has the burden from NCDs. Diabetes and chronic obstructive pulmonary disease (COPD) have experienced the largest change in disease burden or (years living with disability (YLD) (Figure 1A) (5). NCDs have also seen the largest increase in deaths attributable to modifiable metabolic risk factors including blood glucose, blood pressure, cholesterol, diet, and body mass index (BMI) (Figure 1B). From 2007 to 2017, Cambodia experienced substantial increases in NCD risk factors that drive the most death and disability combined (Figure 1C), including high BMI (72% increase from 2007 to 2017), high fasting plasma glucose (39%), high blood pressure (25%), high low density lipoprotein (LDL) cholesterol (21%), dietary risks (17%), alcohol use (15%), and tobacco use (3%) (6). More than half of Cambodians living with diabetes are untreated and even with treatment, only one-quarter are adequately controlled (7). In addition to increasing challenges for public health (e.g., NCDs are key risk factors for leading causes of death like heart disease and stroke) (8), NCDs carry a huge economic burden for LMICs (3). Cambodian government expenditures for NCDs (KHR 343 billion/US $84 million) are just the “tip of the iceberg”, as NCDs can reduce economic output by 5.6 trillion Cambodian riel (KHR) ($US 1.4 billion) due to indirect costs from loss of workforce and reduced productivity and an increased 23% probability of dying prematurely (age 30 to 70) (9).
mHealth, the use of mobile technologies to improve health and healthcare (10), offers a promising approach to reduce NCD burden in LMICs. LMICs are limited in their health systems infrastructure, and mHealth has the potential to strengthen the six building blocks that the World Health Organization (WHO) has identified for a strong health system: service delivery, health workforce, health information, medical products, vaccines, and technologies (11). While underused in health systems infrastructure, LMICs have increasing access to mobile technologies—worldwide cell phone coverage is at 95% (12) and a substantial proportion of people living in poverty have access to mobile phones (13). In Cambodia, 94% report owning their own phone with 99% reachable by phone (14). With mobile subscriptions soon to equal the number of people in the world (15), mHealth is touted for its potential to close the digital divide in low resource settings (16). Digital health technology like smartphones or tablets can also be used to support the health care workforce’s efficiency and quality of care (17).
There is limited evidence on mHealth and eHealth to reduce NCD burden in LMICs. Digital health typically supports four functions: (I) health promotion & awareness, (II) remote monitoring & care support, (III) disease surveillance & outbreak detection, and (IV) decision support system (18,19). Most evidence on digital health in LMICs is to reduce burden from communicable diseases like HIV and tuberculosis and to support maternal and child health outcomes (20). A recent systematic review of digital health for NCD management in LMICs found eight randomized controlled trials (RCTs), each focused on different mHealth interventions and NCDs (18). All but one study found positive effects, however, since the RCTs were so diverse, one cannot conclude the “great potential of mHealth for addressing NCDs in LMICs” has been realized (18). A more recent systematic review (21) found seven additional articles (22-28) on mHealth to improve NCD care in Asian, African and South American LMICs. These studies all used SMS text messages as their mHealth technology and also reported positive improvements in NCD care. Most studies on digital health for NCD have focused primarily on effectiveness rather than implementation, which given the “know-do” gap (29) of limited uptake of evidence-based practices means even less is known about digital health’s potential to improve health in underserved populations. As such, more evidence is needed to understand how digital health can work to reduce the NCD burden in LMICs.
The aim of this paper is to share findings and lessons learned from a process evaluation of mHealth to improve NCD management for persons living with diabetes and/or hypertension in Cambodia. This evaluation aligns well with mHealth’s focused issue on “Digital Health for Hard-to-Reach Populations”. This issue defines hard-to-reach populations as those who are difficult to involve in research or public health programs due to their physical location (e.g., rural communities), social disadvantages (stigmatized), or economic situation (living in poverty). Our study population is Cambodian adults living with chronic conditions who are underserved by their largely rural geographic location, marginalization as persons living with chronic illness, and economic realities as people living in poverty with limited access to financial, educational, health and other resources (4). Furthermore, mHealth journal defines “digital health” as health interventions that involve either hardware or software solutions and services. In this paper, we focus on the following digital health interventions: mHealth (informational, motivational and behavioral voice messages delivered to a Cambodian NGO’s patient network’s mobile phones), and eHealth [tablets for peer educators (PE) to facilitate patient management and community-clinical linkages].
Methods
Context
This study was conducted in Cambodia, a LMIC in Southeast Asia. Cambodia is unique from other LMICs for its devastating history when the Khmer Rouge revolution in the 1970s resulted in the genocide of a quarter of the population (~two million people or even more) through starvation, exhaustion, and mass extermination (30) and Cambodians continued to struggle into the 1980s during a decade of war with Vietnam (31). Since the 1990s, Cambodia has experienced relative peace and slow economic growth, though many continue to live in poverty. As of 2019, the Cambodian population is 15.3 million people (32), and is largely homogenous (over 90% identify ethnically as Khmer or Cambodian) (6). Cambodia is a “lower middle” income country according to 2015 World Bank classifications (33) with a per capita gross national income (GNI) of $1,070 (34). This change in designation from low to lower middle income status belies the fact that 40% of the population continues to live on $2 or less per day, and 80% of Cambodians live in rural areas with limited access to adequate living conditions, economic opportunities and health care (6). Educational attainment is average five years, with most employed persons working in agricultural, industry and service sectors, and almost half (48%) in informal jobs (33).
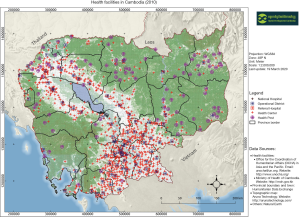
With educated persons such as health professionals targeted during the Khmer Rouge period (35), Cambodia has a significant health workforce shortage of 0.2 doctors, 0.8 nurses and midwives, and 0.04 pharmacists per 1,000 people (36). Like other LMICs, Cambodia spends a small proportion of its GDP on total health expenditures (6% or approximately $70/person) with most of the cost of care born by the population receiving care due to inadequate social health protection mechanisms—61% of health care financing comes from patient’s out of pocket spending (OOP) (37,38). Like other LMICs, Cambodia’s health system is a vertical, rather centralized health system (39) organized for treating acute health care problems, without basics for chronic care. Public health care is distributed through 1,190 local community health centers, 108 regional referral hospitals, 119 health posts, and nine national hospitals (most located in the urban capital of Phnom Penh; Figure 2) (40). The gradual emergence of chronic NCD needs that are unmet by the public system, as well as low government salaries, has contributed to the emergence of over 5,500 more costly private clinics to supplement the lack of public services and provide additional wages for two-thirds of public health staff that work in these clinics (38). And with the indirect costs of NCD care almost 19 times higher than direct costs, the total economic burden of KHR 5.97 trillion (US$ 1.5 billion) is equivalent to 6.6% of the country’s annual GDP, the same percent that Cambodia allocates to health care overall (9).
Setting
MoPoTsyo Patient Information Center (41) was established in 2005 to address the growing number of persons living with diabetes and/or hypertension and the inability of an under-resourced health system designed for acute disease to serve this population. MoPoTsyo’s approach is based on six strategies for improving quality recommended by the Chronic Care Model (CCM) (42): self-management support, decision support, delivery system design, clinical information systems, health care organization, and community resources. MoPoTsyo’s innovative model of chronic care is described in the recent 3rd edition of the World Bank’s Disease Control Priorities (DCP3) (43). Their model aligns with an adapted CCM to account for distinct challenges faced by LMICs—patient-provider communication; service provision at decentralized levels of healthcare; availability of essential medicines for long-term control of blood sugar, blood pressure and lipids; diagnostics and trained personnel; and coordination between the many healthcare providers (44).
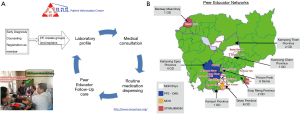
MoPoTsyo trains people living with diabetes and/or hypertension (hereafter referred to as “patients”) to serve as PEs for their community, similar to a lay health or community health worker model, but also as flexible point of care helpers to make actual service delivery work smoothly in often understaffed public environments. PEs provide education and support about NCDs through a self-management book and lifestyle advice posters including Cambodian nutritional advice. They offer important linkages with the health system for regular lab profiles, medical consultations, and routine medication dispensing. MoPoTsyo maintains a centralized database that tracks participants demographics and health outcomes, lab results, doctor’s visits, and pharmacy invoices when patients pick up medications (45). MoPoTsyo’s model addresses financial, geographic, informational and household barriers to NCD care by centering affordable services, medications, and PEs in communities for “comprehensive team-based care closer to home at a reduced cost” [(43) pp. 322]. Figure 3A and 3B illustrate the process by which MoPoTsyo PEs support NCD management and the spread of MoPoTsyo’s PE network in Cambodia.
Study design
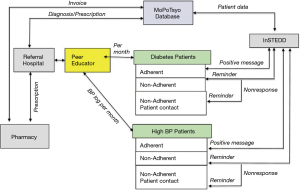
In 2017–2018, we conducted a cluster RCT to develop and test an mHealth communication intervention (voice messaging to mobile phones) and eHealth (electronic data capture by tablet) to improve hypertension and diabetes self-management in Cambodia (46). Seventy-five PEs, representing seven operating districts in rural geographic regions or urban slums, with average 60 patients each, were randomized into 1 of 3 groups—mHealth (mobile voice messages) + eHealth (tablet) only (TMG), tablet only (TG), or no intervention control (CG). We designed the mHealth intervention based on MoPoTsyo patient’s knowledge, attitudes and practices using the Information-Motivation-Behavior Theory framework (47-49), and to respond to previous critiques of mHealth interventions for NCD care management (18). We developed voicemail messages delivered via patient’s mobile phones to provide education about best practices for NCD management (diet/weight management, medications, physical activity, limited use of alcohol and smoking); support for common barriers to NCD management (e.g., acknowledgment that changing diet is difficult when you have limited time and money); and reminders for medication adherence, blood sugar and pressure monitoring, and consultations with their health care providers (50). Mobile voice messages were chosen over mobile SMS/text messages given patient preferences, low literacy and education, and current cell phone technology. We partnered with Innovative Support to Emergencies, Diseases and Disasters (InSTEDD) (51) Southeast Asia to access information from MoPoTsyo’s patient database to tailor and target messages for patients depending on clinical outcomes (e.g., uncontrolled blood pressure or glucose; elevated BMI) and lack of access to services (e.g., had not picked up prescription for 30 days; due for annual check-up with provider). The eHealth intervention provided a computer tablet to 50 PEs to facilitate patient data entry into MoPoTsyo’s database and medical records for longitudinal tracking and access by MoPoTsyo and clinic staff. The aim was to speed up data collection and entry, reduce paper, increase accuracy and quality, and eliminate lengthy distances to bring paperwork to MoPoTsyo from rural provinces. Timely data entry is key for MoPoTsyo’s partnership with pharmacies to provide access to affordable NCD medications, one of the Millennium Development Goals (52). Figure 4 shows how the mHealth and eHealth intervention was designed to facilitate care management, communication and coordination (46).
We conducted a process evaluation to understand if the intervention operated as intended by assessing program operations and whether the target population was served. We will also use this information to guide future digital health research and practice to improve NCD outcomes in LMICs. We used the RE-AIM framework to guide our evaluation, which offers a model for evaluating the public health impact of an intervention (53). Rather than the traditional focus on whether an intervention is effective at improving outcomes of interest, RE-AIM examines other important dimensions for understanding who the intervention reached, the level of intervention uptake, how the intervention was delivered, and whether the intervention was maintained, to better inform future dissemination and implementation of the intervention in real-world settings (54,55). RE-AIM offers a more balanced evaluation of factors related to internal and external validity, and informs practitioners and policymakers on how well a program can reach the target audience, effectively change and maintain outcomes, be adopted and implemented in a range of settings at a reasonable cost, and be sustained over time (56,57). While developed in the U.S. and applied primarily in HICs, RE-AIM has recently been used to guide evaluations in LMICs [e.g., in Mexico (58); in India (59)]. For our process evaluation, RE-AIM is defined as:
❖ Reach: whether the intervention reached the target population, and how representative the study population was of the target population (representativeness);
❖ Effectiveness: whether the intervention was effective at improving health;
❖ Adoption: whether the intervention was adopted across providers (uptake);
❖ Implementation: whether the intervention was delivered as intended (fidelity);
❖ Maintenance: whether the intervention was sustained in routine practice (sustainment).
Data collection and analysis
The study was approved by the University of Washington Division of Human Subjects and the Cambodian National Ethics Committee for Health Research. We used the RE-AIM QuEST mixed methods framework (60) which operationalizes the five dimensions to include both quantitative and qualitative inquiry at multiple levels (patient, provider, context) so the evaluation can identify implementation barriers and facilitators and how the context may influence future translation to other settings. Table 1 shows the quantitative and qualitative research questions that were asked of each RE-AIM domain.
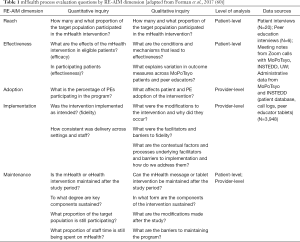
Full table
Data sources included interviews, project team meeting notes, and administrative data. In August 2018, we conducted interviews with 20 study participants to understand how mHealth voice messages were working and opportunities for improvement. We used maximum variation purposive sampling (61) to select a range of participants—received mHealth voice messages over the course of the RCT, received partial messages (received but did not listen to the complete message), did not receive any messages (did not answer the phone when the messages was sent). Furthermore, we included participants who did/did not pick up medications on time, receive a lab test for monitoring blood sugar and pressure, or visit their doctor after receiving mHealth voice messages. We also interviewed 6 of 75 participating PEs to understand their views on how the mHealth patient voicemail messages and the tablets were working. We used criterion-I purposive sampling (61) to identify PEs that had entered</≥40% of patient data into MoPoTsyo’s database using the tablet. We sampled PEs from both rural and urban districts given various access to health care and cellular service in these different areas.
Other data included meeting notes from project team calls with MoPoTsyo (lead Cambodian partner NGO), UW (lead university partner), and INSTEDD (Cambodian consultant to manage the delivery of targeted and tailored voicemail messages to MoPoTso patients) and administrative data from MoPoTsyo and INSTEDD. We extracted a limited dataset from MoPoTsyo’s patient database of RCT study participants in their PE network. INSTEDD provided call logs from the mHealth message delivery, which included data on each message type, duration, and outcome, defined as whether the message was received, listened to partially, listened to multiple times, no answer, or call failed (was never sent due to incorrect number).
We used multiple methods to triangulate the data to strengthen the trustworthiness and rigor of our findings (62). Triangulation can be defined in different ways in mixed methods research—in this study triangulation means the combination of both quantitative and qualitative data to integrate two different ways of thinking about social phenomena to show patterns or idiosyncrasies (63). We used thematic analysis (64) for qualitative data, descriptive statistics for quantitative data, and mixed data sources as appropriate using guidance from Creswell (65) for mixed methods research. Deductive thematic analysis involves the search for and identification of common threads in the data (66) using a pre-existing framework—for our study we used the implementation science framework called RE-AIM (53). We used each of the five RE-AIM constructs to code our qualitative data (interviews and meeting notes) for both latent and manifest content that reflected each theme (66,67). We followed Braun and Clark’s thematic analysis guidance of gathering all data relevant to each theme, creating a thematic map to check that the themes work in relation to the coded text, conducted ongoing analysis to refine the details of each theme and the story the analysis tells, and produce a summary of findings that is shared in this paper (64,68).
For the effectiveness construct in RE-AIM, we used a quantitative statistical method called complier average causal effects model (CACE) to estimate the causal effect of mHealth for those whom actually received the intervention (69,70). CACE models are appropriate to evaluate effectiveness when there is high non-compliance which was seen in our study where 40% of patients in TMG did not receive the messages as intended (due to incorrect mobile phone numbers or service issues). CACE is run using a two-stage least squares model in which randomization is treated as an instrument (71). For the implementation construct in RE-AIM, we used the Consolidated Framework for Implementation Research (CFIR) (72) to categorize determinants by mHealth/eHealth intervention characteristics, the inner setting (organization) and outer setting (e.g., sociopolitical climate). We used Excel (73), ATLAS.ti (74), and Stata (75) for the analysis.
Results
Reach
All of MoPoTsyo patients from the 75 RCT study areas were invited to participate in the study, yet we only required consent from the mHealth participants (TMG) to get their approval for sending messages via their cell phone and to confirm their most current cell phone number. This created three study arms that did not have the same number nor characteristics of participants: 1,737 in the control group (CG), 1,099 in the tablet-only group (TG), and 1,113 in the tablet + messages group (TMG). TG patients had fewer average number of PE visits (15 vs. 29–39 in TMG and CG) and TMG patients had better blood sugar control (73% vs. 80% in TG and CG) and blood pressure control (12% vs. 21% in the other two groups) during the study period. TG and TMG were more urban than rural (66% vs. 44%) than CG. While none of these differences were statistically significant, they reflect clinical differences among patients in each of the groups. Interviews with peers and participants and meeting notes from team planning meetings suggest that for TMG, barriers to participation included frequent changes to their cell phone plans (in which case their number would not work when the INSTEDD system tried to call) and the patients not updating new cell phone numbers with MoPoTsyo. In addition, the requirement to maintain current phone numbers created significant workload barriers to MoPoTsyo staff and PEs. As one PE said, “It is hard for us to keep up with the phone numbers that are always changing so participants can find cheaper plans. We don’t know if they changed their number unless we see them at the next meeting.” (PE 5). This highlighted the need for a system for PEs or patients themselves to regularly update their ever-changing cell phone numbers for mHealth to effectively reach all patients equally.
Effectiveness
In the outcome evaluation, we found no significant improvement in clinical outcomes [systolic blood pressure (SBP), diastolic blood pressure (DBP), or fasting blood glucose (FBG)]. Given the large number of mHealth intervention group participants who did not receive voice messages, we conducted a CACE analysis that compares outcomes for “adherers” (TMG patients who received mHealth voice messages) to “non-adherers” (TMG patients who did not receive messages). We found a clinically though not statistically significant improvement in DBP (2.98 mmHg, 95% CI: −4.6 to 10.5) and FBG (6.6 mg/dL, 95% CI: −2.0 to 15.2) for patients with uncontrolled DBP and FBG and who received all mHealth messages or messages targeted for specific patients (e.g., with high BMI; had not picked up medications before prescription ran out). This suggests mHealth voice messages may be more impactful for patients not currently managing their blood pressure or sugar. As one participant with uncontrolled diabetes and hypertension shared, “When I see MoPoTsyo’s phone number calling me I start thinking, did I remember to take my medications today, did I exercise, have I done what my peer recommends to be healthy?”
Adoption
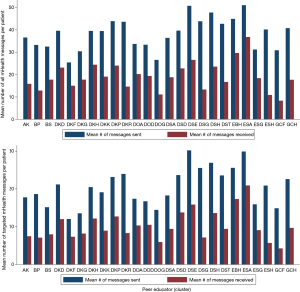
Receipt of mHealth voice messages varied across the 25 PEs in TMG. Looking at all messages, a mean [standard deviation (SD)] of 30 [14] messages were sent (range, 0–51). For targeted messaged only (sent to patients with uncontrolled clinical outcomes and/or other risk factors), a mean [SD] of 15 [7] messages were sent (range, 0–30) and 7 [4] (range, 0–21) were received. Interviews with PEs and patients offered one possible explanation for these wide ranges of message uptake, suggesting patients with frequent relationships with their PE and MoPoTsyo may have been more likely to receive messages by answering the phone when MoPoTsyo called with the recorded message. These patients also had updated phone numbers with the PE which is needed to receive messages. Figure 5 shows the distribution and range of messages sent and received across the 25 PEs. For the eHealth tablets, percent of data being entered via tablet varied from 10–60%.
Implementation
On average, 38% of messages were received across PE clusters and their patients. The main reason for low adherence was many patients in the mHealth group did not receive any messages at all due to an incorrect or invalid mobile number or cell service issues. In addition, many patients worked long hours and shared cell phones with family members so were not able to receive the messages when they were sent (even though messages were programmed to arrive at dinner time when patients would more likely be at home from work with their families). As one participant shared, “I don’t always get the messages because I share a phone with my wife who is at the market all day and we are busy when she gets home from work.” (IW 11). mHealth messages about weight and diet, smoking and alcohol use had the highest hit rate. Both participants and PEs shared they preferred these messages because this is where they are looking for new ways to incorporate lifestyle changes into their lives.
Facilitators to mHealth/eHealth included the strength and quality of the evidence (validity in the intervention being externally developed), the relative advantage (that mHealth/eHealth was an additional form of support and care), adaptability and trialability (patients and PEs were engaged in message development), and complexity (messages were easy to receive). As one participant shared, “Getting a phone message with a familiar voice from MoPoTsyo made me feel like they cared for me which made me want to try what the message advised me.” (IW 7). The tablet was provided with a locally-made synthetic leather protective bright red cover with a foam layer on the inside; yet, 2:25 tablets had to be replaced during the study period. Barriers to the intervention were that mHealth/eHealth were not adaptable once the study had begun and were more complex than initially intended: for the messages, receipt did not equal behavior change, and message delivery was inconsistent due to frequently changing cell phone numbers (and system for tracking this) and shared cell phones among patients and family members; for the tablets, some PEs had difficulty using them regardless of coaching by MoPoTsyo staff and rural PEs were limited by Wi-Fi access despite the provision of mobile hot spots.
At the inner setting (organizational) level, implementation facilitators included structural characteristics (MoPoTsyo is credible as a mature and growing, horizontally structured organization), tension for change (mHealth provides a phone call that PEs would not have time to make and eHealth facilitates data entry and communication), leadership saw mHealth/eHealth as a relative priority and were committed and involved, and compatibility with MoPoTsyo’s values of being an innovative and supportive organization to improve NCD care and outcomes in Cambodia. Barriers were that digital health was not seen as a priority for PEs and those PEs that were not in the eHealth groups wanted tablets to facilitate their work, and that mHealth/eHealth was not able to coordinate with the clinical workflows and systems. While the study had governmental support on the advisory board, the systems were not linked during the trial. As one PE shared, “I though the tablet would make my work easier but instead I had trouble making it work even after MoPoTsyo would call and help, so it became one more thing to manage in my busy day.” (IW 4).
At the outer setting, mHealth/eHealth implementation was facilitated by MoPoTsyo being aware of and trying to align the intervention with known patient and PE barriers to accessing NCD care. Other facilitators include the cosmopolitanism of the MoPoTsyo system (linked with clinics, pharmacies and labs), and external policies and incentives including high cell phone coverage among patients and alignment of the intervention with international and national plans and recommendations for improving NCD care in LMICs. Barriers included the inability of individualized mHealth and eHealth interventions to solve structural barriers to NCD care (e.g., poverty, low education, lack of public transportation), the wide variation in quality of and access to NCD care across PEs’ areas, and the reality that mobile phone coverage and tablets do not equal access and utilization given inconsistent service, frequently changing mobile numbers to save money across multiple network providers, and low education and literacy.
Maintenance
The mHealth and eHealth intervention was not sustained after research grant funding. MoPoTsyo is interested in providing the intervention to support patient members and PEs yet the cost of implementation and maintenance were too high to sustain given the low impact shown by the study. Had the intervention been effective at improving adherence to medications, MoPoTsyo could have maintained funding from extra revenue from the Revolving Drug Fund (45). There is also an unnecessarily high cost of delivering mHealth messages outside the SMART network (with which INSTEDD has a relationship). Cambodia currently has three large cell networks, and costs increase from 2 to 7 cents/message when messages are delivered outside network. In addition, eHealth tablets are expensive to buy and maintain, also given the need for wireless hotspots for the rural PEs. If the intervention was effective then some of these costs could potentially be negotiated down by partnerships between MoPoTsyo and providers. The Ministry of Health is not considering supporting this intervention as it was not universally effective and is being delivered via NGO rather than publicly-funded clinics. MoPoTsyo has been able to repurpose the tablets for maintaining connections with their PE network, using them for video-conferencing training and technical assistance.
Discussion
While digital health interventions were seen as acceptable and appropriate for Cambodians living with diabetes and/or hypertension and the PE network that supports them, they did not result in significant improvements in NCD outcomes in an intent-to-treat analysis. While patients with uncontrolled NCDs who received mHealth voice messages improved their blood sugar and pressure, many were left out. Adoption was a key issue—many patients did not receive mHealth voice messages and many PEs did not utilize eHealth tablets, due to limitations with the cellular network and inconsistent access to mobile numbers and phones.
Our findings align with results from a recently conducted systematic literature review of 30 digital health studies to improve health in developing countries (21). Most studies focused on infectious diseases and maternal health (67%) and used SMS to deliver mHealth messages (60%). Barriers to implementing, sustaining, and scaling digital health interventions were grouped into 14 categories, with the top categories of infrastructure, lack of equipment, and technology gap found across the entire review. Eight of the studies (22-28,76) examined improving NCD care in LMICs (none were from Cambodia or Southeast Asia). Barriers to implementation included technology gap (4:8 studies), lack of or immature infrastructure (3:8), lack of equipment (2:8), cost (2:8), lack of public policy (2:8), psychosocial stressors (2:8), illiteracy (1:8), and lack of efficacy (1:8).
Our study found similar barriers to mHealth to improve management of diabetes and/or hypertension—mobile phone access is different than knowledge on how to use mobile phones for improved NCD management (technology gap); the mobile infrastructure in Cambodia was limited by service blackouts, there are multiple, competing carriers which leads patients to switch phones often, each carrier requires different systems to send mHealth messages (infrastructure); mobile phones remain unaffordable for many patients who share phones with family members (equipment); the lack of affordability of phones, our partner NGO only had affordable access to one service carriers (cost); lack of policies that support and provide funding for mHealth for health care (policy); patients were too busy with work and family life to give time to a phone-based intervention (psychosocial stressors); voice messages were not sufficient to address patients’ limited education and literacy (illiteracy); and mHealth/eHealth was not effective at improving our primary NCD management outcomes of blood pressure or glucose control (efficacy). We did see some improvements for patients with uncontrolled DBP and FBG who received the messages as intended, which aligns with a recent meta-analysis that found mHealth more effective at BP control for people with uncontrolled hypertension (77).
Recommendations for future research and practice of mHealth to improve health outcomes in LMICs align with Kruse and colleagues (21). To overcome barriers, mHealth projects must establish partnerships with local governments, NGOs, clinical systems, and the technology sector to secure funding, leadership and required infrastructure. Engaging and treating hard-to-reach populations living with NCDs in LMICs will require investments in mobile networks and coverage for more stable access and education to improve digital literacy. As with non-digital health interventions, simply building it does not mean people will come; the system needs to enable and support the most vulnerable populations so they have equitable access to the benefits that digital health has to offer. Otherwise, the rise of digital health interventions will only exacerbate the health disparities experienced by marginalized populations.
Furthermore, we know digital health alone may be insufficient for improving clinical outcomes due to structural barriers to accessing care that digital health cannot overcome. While the mHealth community believes mobile phones can transform health and healthcare just as it may help address economic inequities (78), other efforts must be made to address challenges in managing NCDs for hard-to-reach populations, such as lack of access to care in remote areas, limited time and resources for focusing on chronic disease management over basic needs, and limitations in the health system for managing these conditions clinically. While MoPoTsyo has addressed some of these access to care issues with their network of PEs and low-cost medications, laboratory profiles and medical consultations, adding mHealth did not improve quality and effectiveness of that chronic care system. Actual cell phone coverage was closer to 60% for receipt of the mHealth messages compared to the promised “90%” coverage often touted in LMICs.
Conclusions
The global trend of increasing burden of cardiovascular disease in LMICs will continue unless we prioritize ways to effectively engage hard to reach people living with uncontrolled chronic disease (79). Poverty, inequality, lack of education, and the nature of the nutritional transition, are the root causes of the rise in NCD burden in LMICs, and limited resources in these settings means that NCD care competes for political attention and financial investment (46). NCDs also are an important contributor to poverty (80) as they hinder development (81) and disproportionately affect poorer populations in LMICs who have to reply on the inadequate public health system (82). Digital health offers promise for improving NCD management in places where people live and work and where access to health care is limited; however, a realist and systems perspective is needed to develop, deliver and scale digital health tools that close the digital divide and sustainably improve health and well-being of our most marginalized populations.
Acknowledgments
We thank MoPoTsyo’s PEs and patients who participated in our process evaluation. Thank you to MoPoTsyo staff who facilitated participant recruitment. We also wish to thank our in-country study advisory committee: Dr. Chhea Chhorvann (Chair); Dr. Kol Hero, Director of Preventive Medicine Department at Ministry of Health, Ms. Channe Suy; INSTEDD iLab Southeast Asia; Dr. Sok Po, Hospital Services Department, Ministry of Health (MOH); Dr. Touch Khun, Preah Kossamak National Hospital; Dr. Mam Sovatha, National Center for HIV/AIDS, Dermatology, STI at the MOH; Dr. Khim Sam Ath, NCDs and Health through the Life Course (NHL), World Health Organization Phnom Penh.
Funding: This study was funded by the NIH Fogarty International Center and National Institute of Biomedical Imaging and Bioengineering Mobile Health: Technology and Outcomes in Low and Middle Income Countries (R21) 2016–2018 (A Fitzpatrick, PI). The sponsor was not involved in review or approval of the manuscript. Additional funding for in-country data collection and analysis was provided by a University of Washington GO-HEALTH travel fellowship.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Carinne Brody and Sarah Sullivan) for the series “Digital Interventions for Hard-to-reach Populations” published in mHealth. The article was sent for external peer review organized by the Guest Editors and the editorial office.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/mhealth-19-249). The series “Digital Interventions for Hard-to-reach Populations” was commissioned by the editorial office without any funding or sponsorship. They are the Program Manager and Executive Director of the not-for-profit Cambodian NGO MoPoTsyo Patient Information Centre which could influence their views of how the NGO and its work should be portrayed, even though different portrayals have no effect on financial benefits that they receive. AF reports grants from National Institutes of Health, outside the submitted work. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Benziger CP, Roth GA, Moran AE. The global burden of disease study and the preventable burden of NCD. Global Heart 2016;11:393-7. [Crossref] [PubMed]
- Dans A, Ng N, Varghese C, et al. The rise of chronic non-communicable diseases in Southeast Asia: Time for action. Lancet 2011;377:680-9. [Crossref] [PubMed]
- Islam SMS, Purnat TD, Phuong NTA, et al. Non-Communicable Diseases (NCDs) in developing countries: a symposium report. Global Health. 2014;10:81. [Crossref] [PubMed]
- Daniels ME Jr, Donilon TE, Bollyky TJ. The emerging global health crisis: noncommunicable diseases in low- and middle-income countries [Internet]. Independent Task Force Report No. 72, Council on Foreign Relations. December 2014. Available online: https://www.cfr.org/report/emerging-global-health-crisis
- Institute for Health Metrics and Evaluation (IHME). Global burden of disease study [Internet]. Database: GBD Compare. 2018 [cited 10 November 2019]. Available online: http://vizhub.healthdata.org/gbd-compare
- IHME. Cambodia County Profile [Internet]. University of Washington, Seattle, WA. 2018 [cited 10 November 2019]. Available online: http://www.healthdata.org/cambodia
- Otgontuya D, Oum S, Palam E, et al. Individual-based primary prevention of cardiovascular disease in Cambodia and Mongolia: early identification and management of hypertension and diabetes mellitus. BMC Public Health 2012;12:254. [Crossref] [PubMed]
- Gaziano TA. Reducing the growing burden of cardiovascular disease in the developing world. Health Aff (Millwood) 2007;26:13-24. [Crossref] [PubMed]
- United Nations Interagency Task Force on the Prevention and Control of Noncommunicable Diseases, WHO Regional Office for the Western Pacific. United Nations Development Programme. Prevention and control of noncommunicable diseases in Cambodia: The case for investment [Internet]. Prepared for the Ministry of Health of Cambodia. 2019 [cite 10 November 2019]. Available online: http://www10.who.int/nmh/Cambodia-IC-Report-Final.pdf
- Hyder AA, Wosu AC, Gibson DG, et al. Noncommunicable disease risk factors and mobile phones: a proposed research agenda. J Med Internet Res 2017;19:e133. [Crossref] [PubMed]
- World Health Organization (WHO). Everybody's business - strengthening health systems to improve health outcomes: WHO's framework for action [Internet]. WHO: Geneva. 2007 [cited 10 November 2019]. Available online: http://www.who.int/healthsystems/strategy/everybodys_business.pdf
- International Telecommunications Union (ITU). World Telecommunication/ICT facts and figures [Internet]. 2016 [cited 10 November 2019]. Available online: https://www.itu.int/en/ITU-D/Statistics/Documents/facts/ICTFactsFigures2016.pdf
- Kazi AM, Carmichael JL, Hapanna GW, et al. Assessing mobile phone access and perceptions for texting-based mHealth interventions among expectant mothers and child caregivers in remote regions of northern Kenya: a survey-based descriptive study. JMIR Public Health Surveill 2017;3:e5. [Crossref] [PubMed]
- Phong K, Solá J. Mobile phones in Cambodia 2014: Research study [Internet]. Open Institute. 2014 [cited 10 November 2019]. Available online: https://asiafoundation.org/resources/pdfs/MobilephonesinCB.pdf
- Sutcliffe CG, Thuma PE, van Dijk JH, et al. Use of mobile phones and text messaging to decrease the turnaround time for early infant HIV diagnosis and notification in rural Zambia: an observational study. BMC Pediatr 2017;17:66. [Crossref] [PubMed]
- Krishnan A, Ferro EG, Weikum D, et al. Communication technology use and mHealth acceptance among HIV-infected men who have sex with men in Peru: implications for HIV prevention and treatment. AIDS Care 2015;27:273-82. [Crossref] [PubMed]
- McBride B, Nguyen LT, Wiljer D, et al. Development of a maternal, newborn and child mHealth intervention in Thai Nguyen Province, Vietnam: protocol for the mMom Project. JMIR Res Protoc 2018;7:e6. [Crossref] [PubMed]
- Stephani V, Opoku D, Quentin W. A systematic review of randomized controlled trials of mHealth interventions against non-communicable diseases in developing countries. BMC Public Health 2016;16:572. [Crossref] [PubMed]
- Howitt P, Darzi A, Yang GZ, et al. Technologies for global health. Lancet 2012;380:507-35. [Crossref] [PubMed]
- Mbuagbaw L, Mursleen S, Lytvyn L, et al. Mobile phone text messaging interventions for HIV and other chronic diseases: an overview of systematic reviews and framework for evidence transfer. BMC Health Serv Res 2015;15:33. [Crossref] [PubMed]
- Kruse C, Betancourt J, Ortiz S, et al. Barriers to the use of mobile health in improving health outcomes in developing countries: systematic review. J Med Internet Res 2019;21:e13263. [Crossref] [PubMed]
- Leon N, Surender R, Bobrow K, et al. Improving treatment adherence for blood pressure lowering via mobile phone SMS-messages in South Africa: a qualitative evaluation of the SMS-text Adherence SuppoRt (StAR) trial. BMC Fam Pract 2015;16:80. [Crossref] [PubMed]
- Steury EE. Mobile phone short message service to improve malaria pharmacoadherence in Zambia. J Nurs Scholarsh 2016;48:354-61. [Crossref] [PubMed]
- Fang R, Deng X. Electronic messaging intervention for management of cardiovascular risk factors in type 2 diabetes mellitus: a randomised controlled trial. J Clin Nurs 2018;27:612-20. [Crossref] [PubMed]
- Zhou H, Sun S, Luo R, et al. Impact of text message reminders on caregivers’ adherence to a home fortification program against child anemia in rural western China: a cluster-randomized controlled trial. Am J Public Health 2016;106:1256-62. [Crossref] [PubMed]
- Fang R, Li X. Electronic messaging support service programs improve adherence to lipid-lowering therapy among outpatients with coronary artery disease: an exploratory randomised control study. J Clin Nurs 2016;25:664-71. [Crossref] [PubMed]
- Mohan B, Sharma S, Sharma S, et al. Assessment of knowledge about healthy heart habits in urban and rural population of Punjab after SMS campaign-a cross-sectional study. Indian Heart J 2017;69:480-4. [Crossref] [PubMed]
- Rico TM, Dos Santos Machado K, Fernandes VP, et al. Text messaging (SMS) helping cancer care in patients undergoing chemotherapy treatment: a pilot study. J Med Syst 2017;41:181. [Crossref] [PubMed]
- Paken-Walsh N. Learning from one another to bridge the “know-do” gap. BMJ 2004;329:1189.
- Kiernan B. The Pol Pot Regime: Race, Power, and Genocide in Cambodia under the Khmer Rouge, 1975–1979. New Haven: Yale University Press, 1996.
- Morris SJ. Why Vietnam invaded Cambodia: political culture and causes of war. Chicago: Stanford University Press, 1999.
- Ministry of Planning. General Population Census of the Kingdom of Cambodia 2019: Provisional Population Totals. National Institute of Statistics. June 2019.
- World Bank County Dashboards. Cambodia [Internet]. 2016 [cited 10 November 2019]. Available online: http://datatopics.worldbank.org/jobs/country/cambodia
- Ly S. Cambodia is now a lower-middle income country. What does this mean? [Internet]. World Bank. 2016 [cited 10 November 2019]. Available online: https://blogs.worldbank.org/eastasiapacific/cambodia-is-now-a-lower-middle-income-economy-what-does-this-mean
- McLaughlin D, Wickeri E. Mental health and human rights in Cambodia. Fordham Int Law J 2012;35:895-967.
- Koto-Shimada K, Yanagisawa S, Boonyanurak P, et al. Building the capacity of nursing professionals in Cambodia: Insights from a bridging programme for faculty development. Int J Nurs Pract 2016;22:22-30. [Crossref] [PubMed]
- Health Policy Project. Health Financing Profile: Cambodia [Internet]. 2016 [cited 10 November 2019]. Available online: https://www.healthpolicyproject.com/pubs/7887/Cambodia_HFP.pdf
- World Health Organization. Regional Office for the Western Pacific. The Kingdom of Cambodia health system review. Manila: WHO Regional Office for the Western Pacific, 2015. Available online: https://apps.who.int/iris/handle/10665/208213
- Peat S. Cambodia Health System Review: The current policies and strategies of the health system’s governance, financing, and service delivery [Internet]. April 16, 2013 [cited 10 November 2019]. Available online: http://summit.sfu.ca/system/files/iritems1/13979/Peat_Steve-2014.pdf
- Open Development Cambodia (Internet). Health facilities in Cambodia (2010). [cited June 18, 2020]. Available online: https://data.opendevelopmentmekong.net
- MoPoTsyo Patient Information Centre [Internet]. 2019 [cited 10 November 2019]. Available online: https://www.mopotsyo.org/
- Wagner EH. Chronic disease management: what will it take to improve care for chronic illness? Eff Clin Pract 1998;1:2-4. [PubMed]
- Prabhakaran DS, Anand T, Gaziano JC, et al. editors. Cardiovascular, respiratory, and related disorders. Disease Control Priorities, third edition, volume 5. Washington, DC: World Bank, 2017.
- Lall D, Engel N, Devadasan N, et al. Models of care for chronic conditions in low/middle-income countries: a ‘best fit’ framework synthesis. BMJ Global Health 2018;3:e001077. [Crossref] [PubMed]
- van Olmen J, Eggermont N, van Pelt M, et al. Patient-centred innovation to ensure access to diabetes care in Cambodia: the case of MoPoTsyo. J Pharm Policy Pract 2016;9:1. [Crossref] [PubMed]
- Fitzpatrick AL, van Pelt M, Heang H, et al. Using targeted mHealth messages to address hypertension and diabetes self-management in Cambodia: Protocol for a clustered randomized controlled trial. JMIR Res Protoc 2019;8:e11614. [Crossref] [PubMed]
- Fisher JD, Fisher WA. Changing AIDS-risk behavior. Psychol Bull 1992;111:455-74. [Crossref] [PubMed]
- Gao J, Wang J, Zhu Y, et al. Validation of an information–motivation–behavioral skills model of self-care among Chinese adults with type 2 diabetes. BMC Public Health 2013;13:100. [Crossref] [PubMed]
- Osborn CY, Egede LE. Validation of an information-motivation-behavioral skills model of diabetes self-care (IMB-DSC). Patient Educ Couns 2010;79:49-54. [Crossref] [PubMed]
- Steinman L, Heang H, van Pelt M, et al. Facilitators and barriers to chronic disease management and mHealth: a qualitative study to design a mobile phone intervention for people living with diabetes and hypertension in Cambodia. JMIR Mhealth Uhealth 2020;8:e13536. [Crossref] [PubMed]
- InSTEDD (Innovative Support to Emergencies Diseases and Disaster) iLab Southeast Asia [Internet]. 2019 [cited 10 November 2019]. Available online: http://instedd.org/category/cambodia/
- WHO. World health statistics 2010 [Internet]. 2010 [10 November 2019]. Available online: http://www.who.int/whosis/whostat/2010/en/
- Glasgow RE, Vogt TM, Boles SM. Evaluating the public health impact of health promotion interventions: The RE-AIM framework. Am J Public Health 1999;89:1322-7. [Crossref] [PubMed]
- Belza B, Toobert DJ, Glasgow RE. RE-AIM for program planning: overview and applications [Internet]. 1999 [cited 10 November 2019]. Available online: https://www.ncoa.org/wp-content/uploads/IssueBrief_ReAim_Final-2.pdf
- Reach Effectiveness Adoption Implementation Maintenance (RE-AIM) website [Internet]. Available online: http://re-aim.org
- Glasgow RE, Emmons KM. How can we increase translation of research into practice? Types of evidence needed. Annu Rev Public Health 2007;28:413-33. [Crossref] [PubMed]
- Jauregui E, Pacheco AM, Soltero EG, et al. Using the RE-AIM framework to evaluate physical activity public health programs in Mexico. BMC Public Health 2015;15:162. [Crossref] [PubMed]
- Lee RE, Galavíz KI, Soltero EG, et al. Applying the RE-AIM conceptual framework for the promotion of physical activity in low- and middle-income countries. Rev Lat Am Enfermagem 2017;25:e2923. [Crossref]
- Aziz Z, Mathews E, Absetz P, et al. A group-based lifestyle intervention for diabetes prevention in low- and middle-income country: implementation evaluation of the Kerala Diabetes Prevention Program. Implement Sci 2018;13:97. [Crossref] [PubMed]
- Forman J, Heisler M, Damschroder LJ, et al. Development and application of the RE-AIM QuEST mixed methods framework for program evaluation. Prev Med Rep 2017;6:322-8. [Crossref] [PubMed]
- Palinkas LA, Horwitz SM, Green CA, et al. Purposeful sampling for qualitative data collection and analysis in mixed method implementation research. Adm Policy Ment Health 2015;42:533-44. [Crossref] [PubMed]
- Patton MQ. Enhancing the quality and credibility of qualitative analysis. Health Serv Res 1999;34:1189-208. [PubMed]
- Mertens DM, Hesse-Biber S. Triangulation and Mixed Methods Research: Provocative Positions. J Mix Methods Res 2012;6:75-9. [Crossref]
- Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol 2006;3:77-101. [Crossref]
- Creswell JW, Plano Clark VL. Designing and conducting mixed methods research. 3rd edition. Sage Publications, Inc., 2017.
- DeSantis L, Noel Ugarriza D. The concept of theme as used in qualitative nursing research. West J Nurs Res 2000;22:351-72. [Crossref] [PubMed]
- Sandelowski M, Barroso J. Classifying the findings in qualitative studies. Qual Health Res 2003;13:905-23. [Crossref] [PubMed]
- Vaismoradi M, Turunen H, Bondas T. Content analysis and thematic analysis: Implications for conducting a qualitative descriptive study. Nurs Health Sci 2013;15:398-405. [Crossref] [PubMed]
- Little R, Yau L. Statistical techniques for analyzing data from prevention trials: Treatment of no-shows using Rubin’s causal model. Psychol Methods 1998;3:147-59. [Crossref]
- Jo B. Statistical power in randomized intervention studies with noncompliance. Psychol Methods. 2002;7:178-93. [Crossref] [PubMed]
- Angrist JD, Imbens GW, Rubin DB. Identification of causal effects using instrumental variables. J Am Stat Assoc 1996;91:444-55. [Crossref]
- Damschroder LJ, Aron DC, Keith RE, et al. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci 2009;4:50. [Crossref] [PubMed]
- Microsoft. Excel Version 16.1. Seattle, WA: Microsoft Corp., 2017.
- ATLAS.ti. Atlas.ti Qualitative Data Analysis Software. Version 7. Berlin, Germany: ATLAS.ti Scientific Software Development GmbH, 2013.
- StataCorp. Stata Statistical Software: Release 14. College Station, TX: StataCorp LP, 2015.
- Piette JD, Datwani H, Gaudioso S, et al. Hypertension management using mobile technology and home blood pressure monitoring: results of a randomized trial in two low/middle-income countries. Telemed J E Health 2012;18:613-20. [Crossref] [PubMed]
- Lu X, Yang H, Xia X, et al. Interactive mobile health intervention and blood pressure management in adults: a meta-analysis of randomized controlled trials. Hypertension 2019;74:697-704. [Crossref] [PubMed]
- Hall CS, Fottrell E, Wilkinson S, et al. Assessing the impact of mHealth interventions in low- and middle-income countries--what has been shown to work? Glob Health Action 2014;7:25606. [Crossref] [PubMed]
- Bloom D, Chisholm D, Jane-Llopis E, et al. From burden to “best buys”: reducing the economic impact of non-communicable diseases in low- and middle-income countries [Internet]. 2011 [cited 10 November 2019]. Available online: https://www.who.int/nmh/publications/best_buys_summary.pdf
- Beaglehole R, Bonita R, Horton R, et al. Priority actions for the non-communicable disease crisis. Lancet 2011;377:1438-47. [Crossref] [PubMed]
- Beaglehole R, Yach D. Globalisation and the prevention and control of non-communicable disease: the neglected chronic diseases of adults. Lancet 2003;362:903-8. [Crossref] [PubMed]
- Alwan A. Global status report on noncommunicable diseases 2010. Edited by WHO. Geneva, Switzerland: World Health Organization; 2011:176.
Cite this article as: Steinman L, van Pelt M, Hen H, Chhorvann C, Lan CS, Te V, LoGerfo J, Fitzpatrick AL. Can mHealth and eHealth improve management of diabetes and hypertension in a hard-to-reach population? —lessons learned from a process evaluation of digital health to support a peer educator model in Cambodia using the RE-AIM framework. mHealth 2020;6:40.


