A pilot randomized controlled trial (RCT) of daily versus weekly interactive voice response calls to support adherence among antiretroviral treatment patients in India
Introduction
India has the third highest number of people living with HIV (PLH) in the world (1). Among the 36% of Indian adults living with HIV with access to life-saving antiretroviral therapy (ART), most access ART through government-sponsored programs (2). India implemented its free ART roll-out program in 2004, with the goal of attaining >95% adherence rates (3). As of 2016 there were 528 ART Centers in India, serving almost one million PLH (4). Yet, non-adherence among PLH remains high and there is a paucity of research on interventions to improve ART adherence in India. Adherence has been defined as taking 95% of prescribed ART doses (5,6). One 2020 meta-analysis estimated 33% of PLH in India do not reach 95% adherence (7).
HIV requires lifelong self-management to remain adherent. Low adherence and interruptions can lead to viral rebound and negative consequences including transmission to others (8,9), failure of and potentially resistance to first-line medication (10), and HIV disease progression (4). Improving and sustaining ART adherence is essential to both prevention and treatment outcomes. One exploratory qualitative study in India found missed appointments, loss to follow-up, and forgetting doses to be common barriers to ART adherence (11). Forgetfulness is the most common barrier to ART adherence reported in literature (12-15) highlighting opportunities for mobile medication reminders to improve ART adherence.
A systematic literature review with evidence-based recommendations suggested reminder devices and use of communication technologies with an interactive component as a recommended self-management tool for ART adherence (16). Short message service (SMS) reminders and interactive voice response (IVR) calls have also been successful at improving barriers to retention (15,17-22). In 2017, India reached nearly 1 billion mobile phone subscribers with rates of “teledensity”, or the proportion of mobile phones to population, of 172% in urban and 58% in rural areas (UCLA) (23). Using mobile phones to support ART adherence is a low cost, easily diffused, and potentially efficacious intervention strategy (18,24). Two prior studies in India demonstrated patient preferences for IVR calls over SMS, and that IVR calls alone function as adherence reminders (15,25).
The current study was funded by an Indo-US bilateral R21 exploratory/development grant with two broad aims: (I) to document ART adherence rates, behaviors, and related factors; and (II) to pilot test in a randomized controlled trial the efficacy of daily compared to weekly automated IVR calls to support adherence and address related factors such as social support, depression, coping, and self-management. We present the following article in accordance with the CONSORT guideline checklist (available at http://dx.doi.org/10.21037/mhealth-19-248a).
Methods
Study design overview
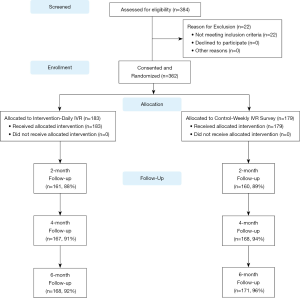
PLH in a large government clinic in Kolkata, India were recruited from April 2014 to March 2015. Among the 384 potential participants screened for the study, 362 (94%) were eligible and enrolled. Eligibility criteria included: 18 years of age, on first or second-line ART regimen, had a CD4 count in last two months (to collect baseline and follow-up CD4 counts prior to the 6-month follow-up), and reporting one or more missed ART doses in the last six month (the research team anticipated strong social desirability biases for under-reporting non-adherence based on feedback from the pilot study participants). Ineligible patients were invited to re-screen when they received their next routine semi-annual CD4 results. Participants were randomly assigned to an intervention or attention control arm, stratified by gender. The intervention arm consisted of twice-daily IVR ART reminder calls with self-management content, three appointment reminders, and a brief weekly IVR adherence and symptom monitoring assessment over a 6-month period. The attentional control arm received the weekly IVR assessment only. The attentional-control design was selected due to ethical considerations resulting from several RCTs showing that weekly text message reminders improved ART adherence (21,26-28). Baseline and follow-up assessments were conducted with high retention at 2 months (89%), 4 months (93%), and 6 months (93%). Two study participants died during the study, unrelated to study procedures. The study protocol was approved by the Institutional Review Boards of the University of California, Los Angeles and the Durbar Ethical Review Committee and was registered with ClinicalTrials.gov (#NCT02118454). Figure 1 shows the study design and participant flow.
Recruitment procedures
Participants were primarily recruited from the ART Centre at the Calcutta School of Tropical Medicine (STM), which hosts the largest ART center in the Northeast Region of India and had over 3,000 active patients on first line ART. Participants were also recruited from the Mamata Care and Treatment Center (MCTC) and the associated Mamata’s Network of Positive Women (MNPW), which provides HIV testing and treatment linkage and support for sex workers and their male partners and social networks. A research team member administered a verbal introduction script to patients in clinic waiting areas. Interested participants were screened in a private area.
Following recruitment and informed consent, participants were randomized within site to either the control or intervention group. Randomization was balanced by gender using an algorithm designed by the UCLA team and implemented in real-time in the mobile phone screening and assessment application by Dimagi Inc. (described below). Interviewers were blind to randomization assignment during the baseline interview. Participants without a personal mobile phone were provided one with service coverage. Participants received a brief training on how to respond to queries from IVR calls.
Intervention piloting and development
Mixed-methods formative research, including a community advisory board (CAB), pilot testing and focus group discussions, informed IVR message development and delivery (26). The CAB refined the secondary aims of the intervention, shifting the focus from drug and alcohol abuse—not considered major problems in the community—to nutrition, hygiene and depression. Focus group discussions highlighted privacy and disclosure-related concerns. Participants expressed concern about involuntary disclosures to others with access to their mobile phones, and did not want messages with HIV or STI-specific content. Similarly, some participants did not want adherence messages to mention ART explicitly and felt that calls alone served as medication reminders. Almost all participants requested messages on general health and wellness (26).
Dimagi, Inc., a social enterprise mobile health technology company with field offices in Delhi, developed and managed the integrated study screening, randomization, assessment, follow-up, and IVR mobile technology platform. Dimagi’s CommCare platform is a HIPAA compliant, cloud-based mobile phone data collection and case management platform. The IVR calls were implemented in India by a gateway provider (Koo Koo). Upon completion of the study, all data was downloaded from CommCare’s server for data analysis and permanently deleted from Dimagi’s servers.
Intervention
Participants randomized to the intervention arm received two IVR calls per day, once in the morning and another in the evening between 9 am and 9 pm to comply with India’s regulations for IVR and SMS gateway providers. Participants were able to choose the times that they would receive calls, ideally to coincide with when they typically took their ART medication to function as an alarm. The IVR message set included 120 messages, each lasting less than 60 seconds, broadly categorized into three domains: medically-related, health promotion, and mental health. Medically-related messages focused on self-management of treatment (adherence, provider communication), symptoms (nausea, dehydration), and co-infections (tuberculosis). Health promotion messages focused on diet, nutrition, and hygiene. Mental health messages focused on self-management strategies for stress reduction, mood improvement, positive cognitions, and social support. Cognitive-behavioral messages were adapted from two RCTs on depression (27) and co-morbid depression and alcohol use (28).
Messages served multiple functions including engagement, intervention framing, information, motivation, and behavioral cues. Message lists were randomized within each category, then domain, and then formed into a queue alternating medically-related, health promotion, and mental health messages so that none of the 120 messages were repeated in a single 2-month period. Messages were available in Bengali and Hindi and in male and female voices to match the participant’s gender to avoid negative reactions from spouses. At the end of each message, participants were asked if they liked the message by pressing 1 (yes) or 2 (no) to assess participant engagement and to inform future intervention content. In the event that a call was unanswered, two more attempts were made (at 5-minute intervals). In addition, three appointment reminder messages were sent at 7-, 2- and 1-day prior to participants’ scheduled HIV appointments, generally monthly or every two months.
Intervention and attention control participants received weekly monitoring IVR calls, which consisted of four questions querying if any ART doses were missed, physical symptoms (side effects), and mental health symptoms (sadness, worry/anxiety) in the last week. Participants responded by pressing 1 (yes) or 2 (no) on their phone’s keypad. In the event of nonresponse to two consecutive weekly IVR assessment calls, follow-up by an interviewer was triggered to maximize retention and simulate clinic staff follow-up. If requested, participants could opt-out of IVR message and/or assessment calls. CommCare also recorded whether IVR calls were answered and their duration.
Assessments
Participants completed assessments at baseline, 2-, 4- and 6-months follow-up using structured questionnaires administered by interviewers using the CommCare platform on mobile phones. Generally, follow-up assessments were scheduled on the same day as ART center visits. Data was also collected from participant medical records for up to 18 months prior to baseline and at each follow-up. ART patients in India are provided an “ART card” to carry to their appointments that include information related to HIV outcomes, including CD4 cell count (conducted once every 6 months) and ART pill counts.
Measures
Three primary outcome measures on antiretroviral medication adherence were assessed, including:
- ART adherence based on pill counts from the ART card, including 6 months of monthly ART pill counts prior to baseline and through 6-month follow-up. Physician-reported pill counts ranged from 0 to 60 pills, with a higher value indicating worse ART adherence;
- Self-reported adherence from the AIDS Clinical Trials Group (ACTG) measures (12) (ex. Did you miss your dose 3 days ago?), including ART self-efficacy (ex. In the last 2 months, when you feel better, have you sometimes stopped taking your medicine?), and beliefs about medication effectiveness (ex. Do you think the HIV in your body will become resistant to ART if you do not take them as prescribed?). Self-reported time since last missed dose was measured using an ordinal variable from the ACTG measure (0 = never or not applicable, 1 = more than 3 months ago, 2 = 1–3 months ago, 3 = 2–4 weeks ago, 4 = 1–2 weeks, 5 = within the past week), which was dichotomized for this analysis into missed last dose within 1 month = 1 versus missed last dose more than 1 month ago =0; and,
- ART Card-verified CD4 cell counts, including up to three CD4 counts to baseline for up to 18-months pre-baseline.
Secondary outcomes were assessed by self-report, including:
- Alcohol Use—the Alcohol Use Disorders Identification Test (AUDIT-C), a brief three-item measure on hazardous alcohol use scored on a scale of 0–12 with a higher score indicative of hazardous drinking habits (29);
- Anxiety and Depressive Symptoms—measured by the 8-item depression subscale of the Hospital Anxiety and Depression Scale (HADS) that has high concurrent validity with diagnostic tools such as the CESD (30). The HADS-D has been used throughout India and translated to Bengali for use with PLH in Kolkata (31). Example survey questions include, “I feel as if I am slowed down,” “I still enjoy the things I used to enjoy,” and “I can sit at ease and feel relaxed”. Responses are reported on a Likert-scale ranging from 0 “Not at all” to 3 “Most of the time” and reverse coded for negatively-framed items. The HADS-D has four scoring levels generated based on the sum of the responses: none [0–7], mild [8–10], moderate [11–14], and severe [15+]. One pilot study with PLH for the current study using this measure found high rates of depression but low rates of anxiety (26). Therefore, the anxiety subscale was excluded in this study to reduce assessment burden;
- Coping Skills—measured by the self-report Brief COPE (32) applied to coping with HIV/AIDS and taking ART. Fourteen domains were assessed with sub-scales consisting of two items each with responses ranging from 0 “Not doing this at all” to 3 “Doing this a lot” and summed to a 0–6 score for each sub-scale domain: self-distraction, active coping, denial, substance use, use of emotional support, use of instrumental support, behavioral disengagement, venting, positive reframing, planning, humor, acceptance, religion, and self-blame. For example, self-distraction is assessed with the items: “I’ve been turning to work or other activities to take my mind off things” and “I’ve been doing something to think about it less, such as going to movies, watching TV, reading, daydreaming, sleeping, or shopping.”
More detailed descriptions of psychosocial measures and secondary outcomes used in this study are presented in a prior publication (26,33). Demographic characteristics of study participants included age, gender, income, educational attainment, profession, number of dependents, relationship status, and HIV status of partner. Age is a continuous variable measured in years. Gender is a dichotomous variable with responses 1 “Woman” versus 0 “Man”. Two transgender women were included in the “Woman” category since they identified as women and mirrored cisgender women on all measured demographic variables. Income is a continuous variable of household monthly income categorized in 100 Indian rupee (INR) increments for analyses. Educational attainment is an ordinal categorical variable measured as highest education level completed with responses 0 “No formal education and illiterate”, 1 “No formal education but literate”, 2 “Class 5”, 3 “Class 10”, 4 “Class 12”, 5 “Graduate”, and 6 “Post-graduate”. Number of dependents is a continuous variable for total number of people relying on the respondent’s financial support ranging from zero to ten people. Relationship status is a nominal variable with responses “Never Married”, “Married”, “Divorced”, “Widowed”, or “Separated”. HIV status of partner is a dichotomous variable with responses 0 “HIV-negative” versus 1 “HIV-positive or not sure”.
Implementation delay and protocol change
A critical challenge and delay in the RCT launch was the change in India’s IVR regulations in September 2013, 1 month before the scheduled launch of this study in October 2013. The Telecom Regulatory Authority of India’s (TRAI) “do not disturb” regulation is a consumer protection measure implemented to limit text-messages and IVR calls from commercial providers to the hours of 9 am to 9 pm. This regulation also includes an “opt-in” or “opt-out” mechanism to be managed by the Telecom providers. The original protocol allowed patients to set their IVR call times outside the 9 am to 9 pm window. Therefore, the study protocol had to be revised and resubmitted to the Institutional Review Boards to accommodate 9 am to 9 pm call times and to incorporate procedures for participants to opt-in or opt-out of IVR calls through their mobile phone service providers. Approvals were received in March 2014, and the study was launched in April 2014, 6 months after the ART clinics expected to start the study.
Statistical data analysis
Baseline descriptive analyses were conducted with simple frequency distribution methods (means, ranges, standard deviations for continuous variables and proportions for categorical variables). Differences between intervention and control groups at baseline were assessed using pooled t-tests for continuous variables and chi-squared tests for categorical variables.
To assess pre-baseline correlates of non-adherence, we examined self-reported time since last missed dose and physician-reported pill counts recorded on patient ART cards prior to baseline. Zero-inflated negative binomial regression was used to model the pill counts to account for over-dispersion and excess zeros.
To assess intervention effects over time unadjusted and adjusted longitudinal analyses were conducted with random coefficient models, a specific subset of the more general multilevel modeling framework, to assess intervention effects for primary adherence outcomes. Coefficient estimates were fit on the logit scale. All statistical analyses were conducted using SAS 9. The sample size supports statistical power to detect responses to intervention (i.e., effect size) of a mean change of 0.40 from baseline to follow-up. A P value of <0.05 was considered to denote statistical significance.
Results
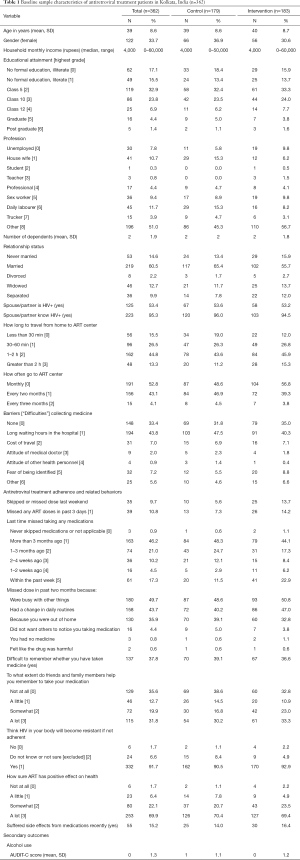
Table 1 provides a summary of sample baseline characteristics. The majority of the sample was male (66.3%) with mean age of 39 years. Approximately 33% of participants had no formal education and 17% were illiterate. The mean number of dependents was two. Approximately 53% of participants had a partner living with HIV. Among those with an HIV-positive partner, 95% reported their partner/spouse knew their HIV-positive status. The mean AUDIT-C score was 0.4 on a 0–12 scale, confirming the CAB’s feedback that alcohol abuse was not a major concern in this community.
Full table
There were modest imbalances between intervention and control groups in a few of the self-reported ART adherence variables at baseline: missing an ART dose last weekend (13.7% intervention vs. 5.6% attention control, P=0.012); missing an ART dose past 3 days (14.2% intervention vs. 7.3% attention control P=0.041); and missing last ART dose within the past week (22.9% intervention vs. 11.9% attention control, P=0.019). Overall, 46% reported last missing dose more than three months ago, 21% reported missing 1–3 months ago, 15% reported missing 1–4 weeks ago, and 17% reported missing any medications within the past week. One in 3 reported finding it difficult to remember whether they took their medication (0= No, 1= Yes). The most common reasons for a missed dose in the past two months were being busy with other things (50%), a change in daily routines (44%), and out of the home (36%). Less than 1% reported missing a dose due to a lack of medication or because they felt like the drug was harmful. More than 60% reported that their friends and family members helped them remember to take their medication.
Baseline correlates of non-adherence
Self-reported missing a dose within one month before baseline was associated with: coping via venting (OR=1.316, 95% CI: 1.084–1.598, P=0.006), coping via self-blame (OR=1.257, 95% CI: 1.001–1.579, P=0.049), severe depression measured by HADS-D score >15 (OR=1.807, 95% CI: 1.023–3.192, P=0.042), AUDIT-C score (OR=1.217, 95% CI: 1.013–1.463, P=0.036), and difficulty remembering to take medication (OR=1.696, 95% CI: 1.037–2.773, P=0.035).
The only significant correlate of physician-reported pill count on patient ART card was difficulty remembering to take medication (b=0.235, 95% CI: 0.075–0.394, P=0.004).
Intention-to-treat analyses
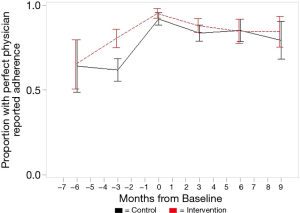
Overall, participants in both intervention and attention control groups improved adherence in the six months before baseline. Figure 2 shows the proportion of patients with perfect adherence from ART cards increased from about two thirds of the sample having “perfect” adherence 6-months prior to baseline, to about 90% at baseline, and then sustained throughout follow-up periods. We tested intervention effects and adjusted for small imbalances at baseline. Mixed models for ART card-based outcomes show very slight negative slopes for intervention by CD4 count (B=−0.000, SE=0.000, P=0.034), percent adherence as a binary variable (B=−0.016, SE=0.002, P<0.001), and perfect adherence (Yes/No) (B=−0.017, SE=0.003, P<0.001). Mixed models for ART card outcomes based on pill counts with covariates show a negative slope for CD4 count and being male (B=−0.352, SE=0.056, P<0.001). The models show a positive slope for percent adherence (binary) and being male (B= 0.670, SE=0.212, P=0.002) and perfect adherence (Yes/No) and being male (B=0.887, SE=0.218, P<0.001). Although estimates may be statistically significant, the small coefficients show the results may not be clinically significant, as evident in Figure 2 by showing the slight decline in adherence from baseline to 6-month follow-up.
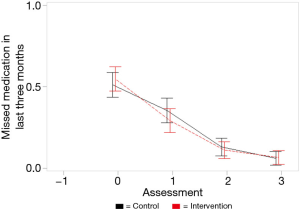
Figure 3 shows similar trends for patient self-reported adherence. At baseline, about half in both groups reported last missing a dose within past three months, decreasing to about 10% of the sample by 6-months. Mixed models show a negative slope for skipping an ART dose in past three months (B=−0.000, SE=0.002, P=0.878; not statistically significant) and for days missed last week measured as binomial distribution (B=−0.001, SE=0.003, P=0.853; not statistically significant).
Discussion
This study aimed to examine the efficacy of automated twice-daily ART reminder calls with health messages compared to once weekly calls to improve or sustain ART medication adherence. Retrospective medical chart data suggest that anticipation of the study and the adherence monitoring involved in the study in conjunction with the 6-month study launch delay may have provided incentives and an opportunity for physicians, nurses, clinic counselors, and patients to increase ART adherence prior to study launch. The high adherence rates at baseline of the RCT resulted in a ceiling effect in which there was little room for further adherence improvements. However, the results do demonstrate adherence improvements were sustained over six months in both groups.
Two other studies in India that were rated as strong quality studies in a systematic review (34) tested the efficacy of mobile phone call reminders to improve ART adherence and found no significant effects. An RCT with HIV-positive patients in south India (n=631) aimed to assess whether weekly IVR reminders improved ART adherence and found no observed differences between the two arms, and suboptimal adherence was similar between both groups at the end of 2 years. Another RCT (n=196) utilizing 3-minute bi-weekly mobile phone calls to improve ART adherence found no significant effect on adherence to treatment or clinical outcomes (35). The lack of significant results suggests the existing national ART roll-out program, system of counselling and clinical management, and adherence monitoring at the clinic-level may be sufficient to mobilize adherence improvements by providers and patients. The high follow-up rates in the current study suggest automated phone calls can be harnessed to help maintain patient follow-up visits and ART adherence.
Limitations and strengths
We acknowledge several limitations inherent in this study. First, based on priorities expressed by the grant peer reviewers and clinic partners in India to assess overall adherence and related factors, and concerns expressed by the field team for under-reporting of non-adherence at screening, the screening criteria threshold was very low at one or more missed dose in the past 6 months. This, combined with increased adherence rates in the 6 months prior study launch associated with delays due to telecom regulatory changes, resulted in a high proportion of participants with near perfect adherence at baseline resulting in ceiling effects with little room for improvement based on intervention exposure. Thus, the results are limited by confounding factors of an attention control, ceiling effects of high baseline adherence rates, Hawthorne effect where patients may have modified their behavior in response to being under observation, and historical bias at the clinical recruitment site in which adherence rates improved significantly over the 6-month period prior to the launch of the study. Lastly, there was an insufficient budget to incorporate biomarkers for adherence or viral load measures (despite requests to NIH and ICMR). In addition to self-reported adherence, the current study utilized pill counts from ART cards, a method with economic and clinical advantages in resource-limited settings; however, this method may be subject to social desirability bias (i.e., pill dumping). The budget also did not allow us to increase sample size for statistical power to include additional study arms (e.g., no call control, twice daily calls without weekly IVR assessments).
This study also had several notable strengths. Follow-up retention rates were very high, between 88% to 96%, which strengthen the study design, feasibility of the research, and the capacities of the field team and clinic partners in conducting high quality research in resource limited settings and difficult to reach populations. More broadly, this study further elucidates adherence self-management related factors for PLH in India. Furthermore, the simple and broadly scalable design of this study creates opportunity for equitable delivery to the large numbers of PLH on ART in India with limited technical and functional literacy. It is our hope that these findings may guide the tailoring of future interventions to incorporate an IVR intervention more directly into ART treatment settings and link data for healthcare providers or peer counselors to follow up when participants are nonresponsive to calls or weekly queries. Future research should screen for higher risk, non-adherent patients for targeted intervention, and work to integrate mobile phone adherence and self-management support interventions into clinical care to inform provider follow-up with patients.
Conclusions
Mobile health technology has garnered increasing interest as a pioneering tool for improving ART adherence. This study has built upon a collective body of work highlighting the utility of mobile phone technologies to positively influence ART adherence more widely and equitably, but the best evidence to date suggests that mobile health tools used in conjunction with healthcare provider follow-up are more effective than automated messaging alone (22). Testing innovative, scalable, robust, and low-cost interventions that will facilitate participant monitoring and follow-up is an important step to improving ART adherence and health outcomes for PLH in India. The results from this study contribute to an empirical foundation for future mobile ART adherence interventions.
Acknowledgments
We would like to acknowledge the participants in this study and the field staff responsible for recruitment and assessment (Madhushree Das, Mousimi Chowdhury, Subharanjan Sinha).
Funding: This research was supported by collaborative funding from the Indian Council of Medical Research (ICMR) grant INDO-US/86/9/20 1O-ECD-II and the U.S. National Institutes of Health grant R21AI094666. The second author was supported by an institutional training grant at the UCLA Semel Institute for Neuroscience and Human Behavior through Award Number T32MH109205 from the National Institute of Mental Health and a grant at the University of California Global Health Institute through Award Number D43TW009343 from the National Institutes of Health Fogarty International Center. Additional support was provided by the UCLA Center for HIV Identification, Prevention and Treatment Services (P30MH58107); the UCLA Center for AIDS Research (P30AI028697); and the UCLA Clinical and Translational Science Institute (UL1TR000124).
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Carinne Brody and Sarah Sullivan) for the series “Digital Interventions for Hard-to-reach Populations” published in mHealth. The article was sent for external peer review organized by the Guest Editors and the editorial office.
Reporting Checklist: The authors have completed the CONSORT reporting checklist. Available at http://dx.doi.org/10.21037/mhealth-19-248a
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/mhealth-19-248a). The series “Digital Interventions for Hard-to-reach Populations” was commissioned by the editorial office without any funding or sponsorship. DS reports grants from National Institute of Allergy and Infectious Diseases, during the conduct of the study. AF reports grants from UCLA Semel Institute for Neuroscience and Human Behavior (T32MH109205), grants from University of California Global Health Institute (D43TW009343), during the conduct of the study. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). All procedures performed involving human participants were in accordance with the ethical standards of the Institutional Review Boards of the University of California, Los Angeles and the Durbar Ethical Review Board in West Bengal, India. Informed consent was obtained from all individual participants included in the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Joint United Nations Programme on HIV/AIDS (UNAIDS). Ending AIDS: Progress Towards the 90-90-90 Targets. 2017.
- Joint United Nations Programme on HIV/AIDS (UNAIDS). UNAIDS Data 2017. 2018.
- Ministry of Health and Family Welfare, Department of AIDS Control. Antiretroviral therapy guidelines for HIV-infected adults and adolescents including post-exposure prophylaxis. 2007.
- National AIDS Control Organization (NACO). Annual Report 2016-17. 2017.
- Boyd MA. Improvements in antiretroviral therapy outcomes over calendar time. Curr Opin HIV AIDS 2009;4:194-9. [Crossref] [PubMed]
- Gilks CF, Crowley S, Ekpini R, Gove S, Perriens J, Souteyrand Y, et al. The WHO public-health approach to antiretroviral treatment against HIV in resource-limited settings. Lancet 2006;368:505-10. [Crossref] [PubMed]
- Chakraborty A, Hershow RC, Qato DM, et al. Adherence to Antiretroviral Therapy Among HIV Patients in India: A Systematic Review and Meta-analysis. AIDS Behav 2020;24:2130-48. [Crossref] [PubMed]
- Kalichman SC. Co-occurrence of treatment nonadherence and continued HIV transmission risk behaviors: Implications for positive prevention interventions. Psychosom Med 2008;70:593-7. [Crossref] [PubMed]
- Quinn TC, Wawer MJ, Sewankambo N, et al. Viral load and heterosexual transmission of human immunodeficiency virus type 1. New Engl J Med 2000;342:921-9. [Crossref] [PubMed]
- Karade S, Chaturbhuj DN, Sen S, et al. HIV drug resistance following a decade of the free antiretroviral therapy programme in India: A review. Int J Infect Dis 2018;66:33-41. [Crossref] [PubMed]
- Joglekar N, Paranjape R, Jain R, et al. Barriers to ART adherence & follow ups among patients attending ART centres in Maharashtra, India. Indian J Med Res 2011;134:954-9. [Crossref] [PubMed]
- Chesney MA, Ickovics JR, Chambers DB, et al. Self-reported adherence to antiretroviral medications among participants in HIV clinical trials: the AACTG Adherence Instruments. Aids Care 2000;12:255-66. [Crossref] [PubMed]
- Bartlett JA. Addressing the challenges of adherence. Journal of Acquired Immune Deficiency Syndromes. 2002;29:S2-S10. [Crossref] [PubMed]
- El-Khatib Z, Ekstrom AM, Coovadia A, et al. Adherence and virologic suppression during the first 24 weeks on antiretroviral therapy among women in Johannesburg, South Africa - a prospective cohort study. BMC Public Health 2011;11:88. [Crossref] [PubMed]
- Rodrigues R, Shet A, Antony J, et al. Supporting Adherence to Antiretroviral Therapy with Mobile Phone Reminders: Results from a Cohort in South India. PLoS One 2012;7:e40723. [Crossref] [PubMed]
- Thompson MA, Mugavero MJ, Amico KR, et al. Guidelines for improving entry into and re-tention in care and antiretroviral adherence for persons with HIV: evidence-based recom-mendations from an International Association of Physicians in AIDS Care panel. Ann Intern Med 2012;156:817-33. [Crossref] [PubMed]
- Joint United Nations Programme on HIV/AIDS (UNAIDS). The Gap Report Geneva: UN-AIDS2014. Available online: http://www.unaids.org/en/resources/documents/2014/20140716_UNAIDS_gap_report
- Schroder KE, Johnson CJ. Interactive voice response technology to measure HIV-related behavior. Curr HIV/AIDS Rep 2009;6:210-6. [Crossref] [PubMed]
- Lester RT, Ritvo P, Mills EJ, et al. Effects of a mobile phone short message service on an-tiretroviral treatment adherence in Kenya (WelTel Kenya1): a randomised trial. Lancet 2010;376:1838-45. [Crossref] [PubMed]
- Pop-Eleches C, Thirumurthy H, Habyarimana JP, et al. Mobile phone technologies improve adherence to antiretroviral treatment in a resource-limited setting: a randomized controlled trial of text message reminders. Aids 2011;25:825-34. [Crossref] [PubMed]
- Mbuagbaw L, Thabane L, Ongolo-Zogo P, et al. The Cameroon Mobile Phone SMS (CAMPS) Trial: A Randomized Trial of Text Messaging versus Usual Care for Adherence to Antiretroviral Therapy. PLoS One 2012;7:e46909. [Crossref] [PubMed]
- Mbuagbaw L, van der Kop ML, Lester RT, et al. Mobile phone text messages for improving adherence to antiretroviral therapy (ART): an individual patient data meta-analysis of ran-domised trials. BMJ Open 2013;3:e003950. [Crossref] [PubMed]
- Highlights of Telecom Subscription Data as on 31st May, 2017 [press release]. 2017.
- Shet A, de Costa A. India calling: harnessing the promise of mobile phones for HIV healthcare. Trop Med Int Health 2011;16:214-6. [Crossref] [PubMed]
- Rodrigues R, Poongulali S, Balaji K, et al. The phone reminder is important, but will others get to know about my illness?’ Patient perceptions of an mHealth antiretroviral treatment support intervention in the HIVIND trial in South India. BMJ Open 2015;5:e007574. [Crossref] [PubMed]
- Swendeman D, Jana S, Ray P, et al. Development and Pilot Testing of Daily Interactive Voice Response (IVR) Calls to Support Antiretroviral Adherence in India: A Mixed-Methods Pilot Study. AIDS Behav 2015;19 Suppl 2:142-55. [Crossref] [PubMed]
- Whittaker R, Merry S, Stasiak K, et al. MEMO-A Mobile Phone Depression Prevention Inter-vention for Adolescents: Development Process and Postprogram Findings on Acceptability From a Randomized Controlled Trial. J Med Internet Res 2012;14:e13. [Crossref] [PubMed]
- Agyapong VI, Ahern S, McLoughlin DM, et al. Supportive text messaging for depression and comorbid alcohol use disorder: single-blind randomised trial. J Affect Disord 2012;141:168-76. [Crossref] [PubMed]
- de Meneses-Gaya C, Zuardi AW, Loureiro SR, et al. Alcohol Use Disorders Identification Test (AUDIT): An updated systematic review of psychometric properties. Psychol Neurosci 2009;2:83. [Crossref]
- Snaith RP. The hospital anxiety and depression scale. Health Qual Life Outcomes 2003;1:29. [Crossref] [PubMed]
- Ghose T, Chowdhury A, Solomon P, et al. Depression and anxiety among HIV-positive sex workers in Kolkata, India: Testing and modifying the Hospital Anxiety Depression Scale. Int Soc Work 2015;58:211-22. [Crossref]
- Carver CS. You want to measure coping but your protocol's too long: Consider the brief COPE. Int J Behav Med 1997;4:92-100. [Crossref] [PubMed]
- Swendeman D, Fehrenbacher AE, Roy S, et al. Gender disparities in depression severity and coping among people living with HIV/AIDS in Kolkata, India. PLoS One 2018;13:e0207055. [Crossref] [PubMed]
- Purnomo J, Coote K, Mao LM, et al. Using eHealth to engage and retain priority populations in the HIV treatment and care cascade in the Asia-Pacific region: a systematic review of literature. BMC Infect Dis 2018;18:82. [Crossref] [PubMed]
- Huang D, Sangthong R, McNeil E, et al. Effects of a Phone Call Intervention to Promote Ad-herence to Antiretroviral Therapy and Quality of Life of HIV/AIDS Patients in Baoshan, China: A Randomized Controlled Trial. AIDS Res Treat 2013;2013:580974. [Crossref] [PubMed]
Cite this article as: Swendeman D, Fehrenbacher AE, Roy S, Ray P, Sumstine S, Scheffler A, Das R, Jana S. A pilot randomized controlled trial (RCT) of daily versus weekly interactive voice response calls to support adherence among antiretroviral treatment patients in India. mHealth 2020;6:35.