
Co-design, co-learning, and co-production of an app for pancreatic cancer patients—the “Pancreas Plus” study protocol
Introduction
Background
Pancreatic cancer is a malignant tumor with a poor prognosis. Despite the therapeutic advances in recent years, mortality remains high at around 92% of patients five years after diagnosis. Such discouraging results can be explained by the fact that most cancers are diagnosed at an advanced stage due to the late and nonspecific clinical manifestations of pancreatic cancer and the absence of specific screening tests (1,2).
The only long-time cure is surgery. Unfortunately, only 20% of pancreatic cancer patients are resectable, and pancreatic surgery is particularly complex and associated with a high risk of complications and postoperative mortality (3). Therefore, due to its complexity, treating pancreatic cancer requires a multidisciplinary approach, by a highly specialized team, in all the phases that characterize the diagnostic-therapeutic path, starting from the initial examinations to the follow-up phases or end-of-life care. Moreover, the treatment path is particularly complex, and it requires several decision-making moments (4).
From a patient-centric perspective, the recent literature has highlighted the need to centralize the coordination of the treatment path and some strategic phases of the same (such as pancreatic surgery) in highly specialized centres (5,6). This strategy also represents a critical point in the recommendations of the European Commission (EC) implemented to increase the treatment of pancreatic cancer and, recently, it was included as a cornerstone for the establishment of the formalized Pancreas Units within the Lombardy Region, Italy, through a “Hub & Spoke” model of care (7).
Rationale and knowledge gap
Currently, in the international context, the centres with outstanding experience are not homogeneously distributed throughout the territory, leading to a health migration involving patients and their families with a relevant expenditure of money, time, and energy.
The centralization of treatments in pancreatic surgery represents, therefore, a fundamental step for improving the patients’ outcomes. Still, it is necessary to build a solid network that allows the patient to reach the centres of reference easily and to be able to continue the treatment closer to home while remaining in frequent contact with the treatment centre, according to the “Hub & Spoke” model.
In this context, the World Health Organization (WHO) has been promoting e-health tools to favour, through integrated organizational models, health prevention and promotion activities and pathways for taking care of patients suffering from chronic pathologies even at a distance (8,9). E-health does not aim to replace traditional health systems but rather to integrate and support them, allowing rapid but traced access to care even for cancer patients who are distant and unable to reach the health facility of reference (10).
In such a scenario, the patient must be considered as an active integral part of the treatment path according to the co-production approach (4,11-14) of oncological care already tested for other tumors, for example, breast cancer (15,16). Co-production is recognized as a valuable strategy to optimize clinical results through active and systematic patients’ engagement in understanding and managing their disease and the care path. However, this strategy is not always easy to apply. There are many barriers that can limit the success of co-production (4). Among these obstacles, there is, in particular, a misalignment among the goals, feelings, and concerns between the patient and the clinical staff, a gap in the background and skills of patients (who may have very different ages and levels of education and knowledge), a lack of culture on the side of clinical staff towards the active involvement of patients in the choices of care and treatment. In such a scenario, non-profit associations stand as strategic allies to bridge the gaps among patients, their families, and healthcare institutions.
Objective
To our knowledge, no co-production programmes have yet been devoted to pancreatic cancer patients (15), while experiences have been described about shared-decision making (4). Still, pancreatic cancer patients need to face a complex disease, and dedicated tools must be developed from a patient’s perspective.
Starting from these premises, to increase the continuum process of care, the integration of different professional figures and the patient’s active collaboration, the association “Unipancreas”, based in Italy, has partnered with a multidisciplinary team of professionals with different expertise to develop a project called “Pancreas Plus”. Unipancreas is a non-profit entity devoted to spreading knowledge and supporting patients starting from the pancreatic cancer diagnosis. Unipancreas organizes events to connect patients and healthcare professionals in the field, with the goal of bridging the knowledge gaps that may constitute barriers in the care journey. Following its mission, Unipancreas has conceived the Pancreas Plus project to create an entirely co-designed app to support pancreatic cancer patients, merging the clinicians’ technical knowledge and the patients’ emotions and unmet needs. The realization of the project requires the active cooperation of various actors, each with different knowledge and backgrounds, both in terms of education and emotional involvement, to develop a technological yet easy-to-use tool that can assist patients during their care journey, from the initial tests and diagnosis throughout the treatment, up to the follow-up or end-of-life care.
The “Pancreas Plus” case can serve as an example of co-design, co-learning, and co-production in oncological care. Once the “Pancreas Plus” project is realized, the secondary step in our timeline will be to check the real benefits for pancreatic cancer patients of this multi-stakeholder engagement app in term of remote symptom control, hospital readmission, glycemic control, rehabilitation and nutritional assessment comparing patients app-user and patients who are not.
In December, 2022, the “Pancreas Plus” initiative was awarded by the Roche Foundation within their competitive grant devoted to projects aimed at patients’ support in oncology.
The article reports the study protocol, based on the most recent literature on the topics of pancreatic cancer, e-health, co-production of care, and co-deign of e-health tools.
Methods
The “Pancreas Plus” study protocol and programme sees different steps, defined as follows:
- A preliminary phase, namely the definition of a stakeholders map;
- Four project phases:
- Co-assess;
- Co-learn;
- Co-design;
- Co-validate and co-deliver.
According to Italian laws, non-interventional studies do not necessarily require approval by an ethics committee. In particular, in the panel, the participants will only share their personal opinion about the process. No patients’ data will be ever shared. Eventual additional studies, once the app is created, will be subjected to the preliminary approval of the Ethics committee. The study will be conducted following the principles of the Declaration of Helsinki (as revised in 2013).
The preliminary phase: the definition of a stakeholders map
A preliminary study was conducted to allow Unipancreas to identify and map the main stakeholders that could cooperate in defining the project (17), also in accordance with previous experiences (15,16) as follows:
- Multidisciplinary medical teams with specific skills in the pancreatic field in determining the treatment path and in carrying out treatments (oncologists, surgeons, radiologists, radiotherapists);
- Nursing staff, who represent the first interface with the patients in the management of their daily problems, and who will act as “case managers”;
- Multidisciplinary clinical rehabilitation teams (nutritionists, physiotherapists, physiatrists, psychologists, palliative care specialists) that support the patient from a psycho-physical point of view in the rehabilitation phase, follow-up, or in end-of-life palliative care;
- Cancer patients, former cancer patients, their families, and caregivers, who have a wealth of personal experiences and fears;
- Non-profit associations that can act as disseminators.
In addition, the following actors were also identified as relevant ones to be involved:
- Researchers and research institutes related to oncological research, with particular reference to pancreatic cancer (researchers active in basic research and experimental treatments, pharmaceutical companies, universities, scientific societies…) for the management of data collection for research purposes;
- Mass media linked to the world of health (online portals, television channels, magazines…) for the dissemination of the programme and its results and awareness raising;
- Public bodies (healthcare institutions, regional and national health systems…) for local support, dissemination of the programme and its results, and awareness raising.
The main stakeholders are summarized in the following Table 1.
Table 1
| Who | Why | What & how | |||
|---|---|---|---|---|---|
| Multidisciplinary medical team | Oncologists, surgeons, radiologists, radiotherapists | Competences in pancreatic cancer care | Understanding and management of the treatment paths | ||
| Nursing staff | Nurses | Competencies in patient’s management throughout the care path | Closeness to the patient throughout the treatment journey | ||
| Multidisciplinary clinical rehabilitation team | nutritionists, physiotherapists, physiatrists, psychologists, palliative care specialists | Competencies in rehabilitation, follow-up, and end-of-life palliative care | Understanding and management of the rehabilitation, follow-up, and palliative care paths | ||
| Users | Pancreatic cancer oncological patients, former patients, patients’ families, caregivers | First-hand experience about the personal physical and psychological needs | Understanding and mapping the personal physical and psychological needs | ||
| No profit associations | Patients’ associations, associations that support patients, their families, and research | Knowledge about the local communities and the relevant actors in the scenario | Supporting the patients and disseminating the project’s results | ||
| Researchers and research institutions | Researchers and research institutions, private companies, universities, scientific societies | Competencies in the research gaps and needs and aggregate data management | Identification of the research avenues and data collection and analysis | ||
| Mass media in the field of healthcare | Online portals, television channels and programs, magazines… | Knowledge of a wider audience (clinicians, citizens, companies, institutions, public bodies) | Dissemination of results and awareness raising | ||
| Public bodies | Healthcare institutions, hospitals, regional and national healthcare systems | Local presence, contacts with citizens and other stakeholders | Local support, dissemination of results and awareness raising | ||
Project phases
The project has four distinct phases, which are later described following the co-production framework of Elwyn et al. (11), as reported also by other collective experiences of multi-stakeholder engagement (18).
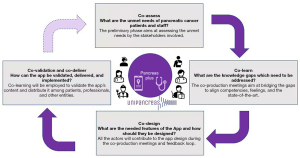
Co-assess
The “co-assess” and preliminary phase of the project aims to address the following question: “What are the unmet needs of pancreatic cancer patients and staff?” The phase concerns a scientific study aimed at testing the perceptions and unmet needs of the main stakeholders with respect to the co-production dynamics. In particular, the phase investigates the relationship between patients and the other actors within the healthcare ecosystem (15).
The objective of the preliminary phase is to understand the expectations and problems of each category of stakeholders to take them into account in the subsequent steps of project design, validation, and training.
Data collection is carried out through questionnaires (surveys) and/or semi-structured interviews starting from the recent literature on patient engagement.
Co-learn
The second phase of co-learn addresses the following question: “What are the knowledge gaps which need to be addressed?” At least one representative from each stakeholders’ category is invited to join a series of in-presence or online meetings. Every stakeholder is asked to share their background and feelings concerning pancreatic tumors. A co-training and co-learning path will be essential to fill the gaps and translate knowledge among the various actors (16,19,20). The training journey aims to bridge the major gaps about the disease, meaning its features, treatment opportunities, the most recent scientific evidence, and the main shortcomings and emerging needs in the current management of patients according to different points of view, identifying some possible solutions.
In consideration of the limitations deriving from the different backgrounds and the results of the preliminary phase, the use of various knowledge translation tools is envisaged (14,21), including graphic design tools (power points, images, sketches, videos or easy-to-understand flowcharts…), but also of a more “soft” type (including the identification of a facilitator or mentor, debates, Q&A - questions and answers…) (14,21,22).
Co-design
Starting from the outcomes of the co-learning phase, stakeholders are guided by the technical team to co-design the shared development of a dynamic interface (app) capable of satisfying the requests and wishes of patients, healthcare professionals, and researchers. More in detail, the stakeholders need to address the following issue: “What are the needed features of the app, and how should they be designed?”
A preliminary study of the existing e-health tools has allowed identifying the main basic features of the new app, including:
- Easy accessibility to all users, including those with basic technological and digital skills;
- Modern, with the possibility of implementing artificial intelligence (AI) and machine learning algorithms;
- Interactive, with voice recognition responder;
- Connected with remote monitoring tools (like smartwatches and wearables);
- Respectful of privacy and security paradigms;
- Able to keep track of the e-services performed.
The tentative content of the app to be discussed with the co-production team members has been defined as reported in the following Table 2.
Table 2
| Step | Objective | Content |
|---|---|---|
| 1 | Prevention | Information on risk factors (nutrition, smoking, alcohol abuse); familiarity information |
| 2 | Starting and planning care | Information on the disease, the course of treatment and the centers of reference (highly specialized hubs versus spokes) |
| 3 | The therapeutic phase | Easy access to a specialist consultation at a highly specialized center by the patient or by local doctors who can act as intermediaries in sharing radiological and clinical examinations with the specialized center |
| Pre-qualification courses: personalized programs that can be implemented at home through periodic supervision by experienced staff to improve the psycho-physical condition before the start of the therapeutic path | ||
| Patient education with personalized programs | ||
| Automatic alerts that incentivize the patient to perform the prescribed exercises | ||
| Pedometer and heart rate monitor (smart-watch) on loan for use to the patient with automatic recording of the activity on the app | ||
| Periodic telephone follow-up by healthcare personnel | ||
| Possibility, through the computer interface, of sharing documentation between specialists involved in patient care to encourage a multidisciplinary approach (oncologists, surgeons, radiologists, physiotherapists, nutritionists, psychologists) | ||
| 4 | The rehabilitation and follow-up phase | Home rehabilitation via: tele-monitoring of vital signs at home |
| Home rehabilitation programs: patient education with personalized programs | ||
| Automatic alerts that incentivize the patient to perform the prescribed exercises | ||
| Pedometer and heart rate monitor (smart-watch) on loan for use to the patient with automatic recording of the activity on the app | ||
| Periodic telephone follow-up by healthcare personnel | ||
| Nutritional support at home: ability to record meals, calories, and diet-related disorders Involvement of family members to allow a double check on actual food intake | ||
| Nutritional advice that takes into account the reported disorders | ||
| Advice on possible therapeutic additions (e.g., insulin therapy, enzymatic supplementation in case of steatorrhea) | ||
| Remote symptom control: possibility of recording symptoms with relative frequency and intensity (using the VAS scale) | ||
| Creation of Alerts of varying severity based on the intensity and frequency of symptoms | ||
| Telemonitoring: periodic monitoring and recording of the parameters of patients discharged at home after surgery using portable digital tools provided on loan for use to the patient (e.g., monitoring body temperature, blood sugar, vital parameters and weight) | ||
| Periodic video consultation for drains and surgical wound evaluation by a professional nurse dedicated to the project |
VAS, visual analogue score.
Co-validation and co-deliver
The last phase aims to co-validate and co-deliver the final app, addressing the following question: “How can the app be validated, delivered, and implemented?” The validation of the app can come only after the sharing of the final result among all team members through different phases, with the possibility of carrying on changes. The evaluation of the platform is carried on in progress through the feedback provided by patients and other stakeholders.
All members of the co-production team need to be trained and tutored by Unipancreas staff and volunteers for optimal use of the app and any associated devices. Training and co-learning are also extended to clinicians, partner institutions, and non-profit entities, and dedicated tools will be developed to disseminate the initiative, like billboards and leaflets in hospitals, cancer support centers, and at dedicated events organized by associations and public bodies. Social media channels of partner institutions are also used for the dissemination of the initiative and the training events.
The phases of the project are summarized in the following Figure 1.
Discussion
The co-production journey leading to the development of a dedicated app is expected to bring numerous benefits, both for the patients and the other stakeholders. Such benefits include the opportunity to serve the patient according to his/her needs while, at the same time, collecting valuable medical information about the pathology and the clinical process.
The co-design of the app has the aim to assess the patients’ unmet needs and address them by offering a range of information about the treatment process and services. The specific content and options will be co-decided by the multiple stakeholders involved while designing the app, according to the defined needs and wishes.
Consultations by experienced clinicians are today possible even at a distance, and the app will allow patients to keep and nourish the progress of their medical data, to be shared with the multidisciplinary team in charge. Data can also be monitored by physicians to optimize the performance status of the candidate patient undergoing surgery or chemotherapy. The e-health functions, especially when connected with monitoring devices (like smartwatches and wearables) will allow keeping track of the patient’s progress and reduce hospital’s access when not necessary, also in a virtual hospital model of care (23,24).
All these hypothesized benefits will be monitored with standard measurement tools, parameters, and indexes. For instance, the team is planning to use QoL questionnaires (EORTC C-30), nutritional assessment in all disease’s steps, and to monitor ratios like hospital stay from surgery, re-admission rate, time from surgery to adjuvant chemotherapy, adherence to chemotherapeutic schedules, time to return to work.
While multiple stakeholder engagement may be challenging due to the different expertise, background, goals, and feelings, several knowledge translation tools (21) will be employed to make the process as smooth as possible.
The project team will keep track about all the steps and actions, including the facilitators used, the issues raised, and the adopted solutions, so that the best practices and lessons learned can be beneficial in other contexts, with different pathologies or aiming at reaching different outcomes. The experience will be disseminated through scientific as well as practice publications, but also through events and clinical meetings with social media coverage.
All in all, the value of the project stands in employing, for the very first time, a co-production approach to merge the different needs and points of view of the actors involved in pancreatic cancer care for the benefit of patients. While the project’s expected primary outcome is a practical e-health tool that may be beneficial for patients but also for clinicians and researchers, we hope to consolidate a methodology that can successfully apply a co-design, co-learn, and co-production framework to other diseases.
Acknowledgments
Funding: None.
Footnote
Peer Review File: Available at https://mhealth.amegroups.com/article/view/10.21037/mhealth-22-48/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://mhealth.amegroups.com/article/view/10.21037/mhealth-22-48/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. According to Italian laws, non-interventional studies do not necessarily require approval by an ethics committee. In particular, in the panel, the participants will only share their personal opinion about the process. No patients’ data will be ever shared. Eventual additional studies, once the app is created, will be subjected to the preliminary approval of the Ethics committee. The study will be conducted following the principles of the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Barcellini A, Peloso A, Pugliese L, et al. Locally Advanced Pancreatic Ductal Adenocarcinoma: Challenges and Progress. Onco Targets Ther 2020;13:12705-20. [Crossref] [PubMed]
- Frassini S, Calabretto F, Granieri S, et al. Intraperitoneal chemotherapy in the management of pancreatic adenocarcinoma: A systematic review and meta-analysis. Eur J Surg Oncol 2022;48:1911-21. [Crossref] [PubMed]
- Gemenetzis G, Groot VP, Blair AB, et al. Survival in Locally Advanced Pancreatic Cancer After Neoadjuvant Therapy and Surgical Resection. Ann Surg 2019;270:340-7. [Crossref] [PubMed]
- Griffioen IPM, Rietjens JAC, Melles M, et al. The bigger picture of shared decision making: A service design perspective using the care path of locally advanced pancreatic cancer as a case. Cancer Med 2021;10:5907-16. [Crossref] [PubMed]
- Balzano G, Guarneri G, Pecorelli N, et al. A four-step method to centralize pancreatic surgery, accounting for volume, performance and access to care. HPB (Oxford) 2021;23:1095-104. [Crossref] [PubMed]
- Balzano G, Guarneri G, Pecorelli N, et al. Modelling centralization of pancreatic surgery in a nationwide analysis. Br J Surg 2020;107:1510-9. [Crossref] [PubMed]
- Previtali P, Dal Mas F, Denicolai S, et al. A multidisciplinary approach to care. A Delphi consensus on the case of Pancreas Units. In: Martellucci J, Dal Mas F, editors. Towards the Future of Surgery. Cham: Springer, 2023.
- Marques ICP, Ferreira JJM. Digital transformation in the area of health: systematic review of 45 years of evolution. Health Technol (Berl) 2020;10:575-86. [Crossref]
- WHO. Using e-health and information technology to improve health Health Topics. 2022 [cited 2022 Dec 5]. Available online: https://www.who.int/westernpacific/activities/using-e-health-and-information-technology-to-improve-health
- Tripepi M, Pizzocaro E, Giardino A, et al. Telemedicine and Pancreatic Cancer: A Systematic Review. Telemed J E Health 2022; Epub ahead of print. [Crossref] [PubMed]
- Elwyn G, Nelson E, Hager A, et al. Coproduction: when users define quality. BMJ Qual Saf 2020;29:711-6. [Crossref] [PubMed]
- Petersson C, Batalden P, Fritzell P, et al. Exploring the Meaning of Coproduction as Described by Patients After Spinal Surgery Interventions. Open Nurs J 2019;13:85-91. [Crossref]
- Batalden M, Batalden P, Margolis P, et al. Coproduction of healthcare service. BMJ Qual Saf 2016;25:509-17. [Crossref] [PubMed]
- Dal Mas F, Biancuzzi H, Massaro M, et al. Adopting a knowledge translation approach in healthcare co-production. A case study. Manag Decis 2020;58:1841-62. [Crossref]
- Cobianchi L, Dal Mas F, Massaro M, et al. Hand in hand: A multistakeholder approach for co-production of surgical care. Am J Surg 2022;223:214-5. [Crossref] [PubMed]
- Bednarova R, Biancuzzi H, Rizzardo A, et al. Cancer Rehabilitation and Physical Activity: the "Oncology in Motion" Project. J Cancer Educ 2022;37:1066-8. [Crossref] [PubMed]
- Johnson AK, Choi SW. Stakeholder engagement, proper planning and modular design for mHealth apps: lessons from QuestExplore and working toward standards for mHealth app design. mHealth 2022; Available online: https://mhealth.amegroups.com/article/view/103756
- Miceli L, Dal Mas F, Biancuzzi H, et al. Doctor@Home: Through a Telemedicine Co-production and Co-learning Journey. J Cancer Educ 2022;37:1236-8. [Crossref] [PubMed]
- Dal Mas F, Biancuzzi H, Massaro M, et al. Knowledge translation in oncology. A case study. Electron J Knowl Manag 2020;18:212-23. [Crossref]
- Graham ID, Logan J, Harrison MB, et al. Lost in knowledge translation: time for a map? J Contin Educ Health Prof 2006;26:13-24. [Crossref] [PubMed]
- Dal Mas F, Garcia-Perez A, Sousa MJ, et al. Knowledge Translation in the Healthcare Sector. A Structured Literature Review. Electron J Knowl Manag 2020;18:198-211. [Crossref]
- Dal Mas F, Bagarotto EM, Cobianchi L. Soft Skills effects on Knowledge Translation in healthcare. Evidence from the field. In: Lepeley MT, Beutell N, Abarca N, et al. (editors). Soft Skills for Human Centered Management and Global Sustainability. New York: Routledge, 2021:95-109.
- Zahedi FM, Zhao H, Sanvanson P, et al. My Real Avatar has a Doctor Appointment in the Wepital: A System for Persistent, Efficient, and Ubiquitous Medical Care. Inf Manag 2022;59:103706. [Crossref]
- Moore G, Du Toit A, Jameson B, et al. The effectiveness of ‘virtual hospital’ models of care: a Rapid Evidence Scan The effectiveness of Virtual Hospital models of care. Sydney, 2020. Available online: https://www.saxinstitute.org.au/wp-content/uploads/20.04_Rapid-Evidence-Scan_The-effectiveness-of-virtual-hospitals.pdf
Cite this article as: Cobianchi L, Dal Mas F, Pizzocaro E, Tripepi M, Butturini G. Co-design, co-learning, and co-production of an app for pancreatic cancer patients—the “Pancreas Plus” study protocol. mHealth 2023;9:16.


