
Mobile health promotion of human immunodeficiency virus self-testing in the United States
Introduction
In 2015, the Centers for Disease Control and Prevention (CDC) estimated that over a million people were living with the human immunodeficiency virus (HIV) infection (1). As of 2017, there were approximately 39,000 new infections, with young adults under 35 years old comprising half of these cases (1). Furthermore, the HIV epidemic disproportionately affects communities of color and gay/bisexual men (1).
To address the HIV epidemic, the United States National HIV/AIDS Strategy seeks to achieve the following by 2020: at least 90% of people living with HIV infection know their HIV status; at least 90% of people diagnosed with HIV infection receive appropriate medical care; at least 80% of people diagnosed with HIV infection are virally suppressed (2). Despite efforts, the United States has yet to attain those goals, as 86% of people living with HIV infection know their status, and 60% of those living with HIV infection have viral suppression (3).
HIV self-testing could increase people’s awareness of their HIV serostatus. Compared with traditional facility-based HIV testing, self-testing has the potential to provide rapid results in private settings. It may also reduce testing barriers, (e.g., stigma, discrimination, and loss of privacy and confidentiality) (4-6). Thus, HIV self-testing may promote frequent testing among high-risk populations (e.g., men who have sex with men) (7). In terms of acceptability and feasibility, studies suggest populations in both high and low-resource settings have been able to accurately perform HIV self-testing with minimal support from trained staff members (8).
Currently, OraSure’s OraQuick In-Home HIV Test is the only HIV self-testing platform approved by the U.S. Food and Drug Administration (9). It detects antibodies to HIV on oral fluid samples with 92% sensitivity and 99.98% specificity (10,11). Since self-testing serves as a screening rather than a diagnostic tool, individuals with positive HIV self-test results have to undergo confirmatory laboratory testing. Due to the “window period,” those with negative HIV self-testing results who may be exposed to HIV in the past 6–12 weeks should consider retesting (12).
Mobile health (mHealth) encompasses medical and public health efforts that utilize mobile wireless technology (e.g., smartphones, tablets) (13). Given mobile wireless technology’s ubiquitous presence, mHealth interventions delivered through existing and popular online platforms (e.g., social media sites, dating apps) used by high-risk populations could promote HIV self-testing and prevention among these communities. mHealth can also address concerns surrounding HIV self-testing (e.g., necessity of confirmatory testing, inadequate follow-up care) by using technology to assist in HIV self-testing distribution, pre- and post-test counseling, and linkage to follow up care (14-16).
We conducted a narrative review of intervention studies and public health programs in the United States. Our goal was to synthesize the role of technology in supporting HIV self-testing and provide recommendations around mHealth and HIV self-testing.
Methods
We conducted a narrative review by searching for PubMed studies between December 2002-January 2019 using the following search phrase: HIV AND prevention AND (self-testing OR self-test OR “self-test” OR “Self test” OR “home test” OR home-testing OR “home testing” OR “home based testing” OR “home-based testing”) AND (mHealth OR “mobile phone” OR “cell phone” OR smartphone OR app OR application) NOT (PrEP OR “pre exposure”). We also used the Google Scholar and Google search engines to find additional studies and public health programs in the United States through January 2019. We included previous and ongoing studies published in English and conducted in the United States in which participants received HIV self-testing kits as part of the mobile health intervention. Studies and programs were categorized based on the means they used technology to promote HIV self-testing uptake.
Results
Nine research studies and two public health programs fit the inclusion criteria and are discussed in this review (Table 1).
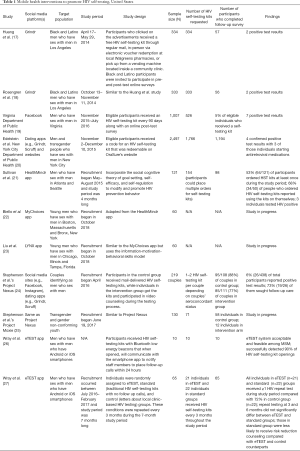
Full table
Advertising on social media to distribute HIV self-testing kits
We identified two studies and two public health programs that leveraged the popularity of social media or dating apps to promote HIV self-testing using advertisements.
In 2016, our team published two studies that used Grindr, a dating app, to distribute free HIV self-tests in high HIV incidence areas in Los Angeles, California (17,18). The team placed full-screen/blast advertisements in Grindr, and visitors who clicked on the advertisements were directed to the study website where they could request a free test kit. Participants could select to receive their self-testing kits through regular mail, in-person via electronic voucher redemption at local Walgreens pharmacies, or pick up from a vending machine located inside a community clinic. Eligible individuals [i.e., Black/African American or Hispanic/Latino men who have sex with men (MSM) aged 18 years or older] were invited to complete pre- and post-test surveys.
Both studies reported similar numbers of HIV self-testing requests (n=334) (17), (n=333) (18) with the majority ordering mailed tests (17, 18). Among eligible participants who completed baseline surveys (n=122) (17) (n=125) (18), many of them engaged in high-risk behaviors (e.g., condomless anal sex in the past 3 months, never or not recently testing for HIV). Self-reported HIV self-testing results were only available for a small number of participants who completed the post-test survey (n=57) (17) (n=56) (18). Among participants who responded to the post-test survey, a majority felt the HIV self-testing experience was easy. In each study, participants who reported a preliminary positive HIV test result (n=2) (17) (n=2) (18) subsequently sought follow up care. Although these studies suggest the potential for social networking sites in promoting HIV self-testing among high-risk populations that will seek follow up care if tested positive, it is important to note that the small follow-up sample size limits the generalizability of the results.
Two state public health departments have implemented social media mHealth-based HIV self-testing distribution interventions to increase HIV testing and prevention among high-risk populations. Designed to target MSM, the Virginia Department of Public Health placed advertisements for free HIV self-testing kits on Facebook from November 2015–July 2016 (19). Eligible MSM participants received an HIV self-testing kit every 90 days along with an online post-test survey. During the first phase of the intervention, 1,007 individuals completed the survey and 526 eligible people were recruited. Among the latter group, 56% had suboptimal HIV testing behaviors (e.g., had not been testing for >1 year or had never received such testing before). Despite the low post-survey participant response rate (5%), 7 individuals reported a positive test result. Data from the second phase of the intervention have yet to be published, but based on the preliminary results, this program has the potential to increase HIV testing among high-risk, untested populations.
To address the New York City (NYC) HIV epidemic among men and transgender people who have sex with men (MTSM), the city’s Department of Health partnered with OraSure to advertise free HIV self-testing kits on popular dating apps (e.g., Grindr, Scruff) and websites (20). Eligible MTSM received a redeemable code for an HIV self-testing kit ordered on OraSure’s website. The kit included information about HIV testing/prevention and follow-up care referrals. Within a month (November 2–December 10, 2015), the team distributed 2,497 test codes. Among recruited participants, 72% were <35 years old, 30% were Hispanic, 11% were Black, and 42% reported not testing for >1 year or never tested before. Within two months, 71% of the participants (1,766/2,497) used the code and 48% (1,194/2,497) completed the follow-up survey. Among the seven participants who reported a positive test result, four of them had a positive confirmatory result. Three individuals who had a positive confirmatory result began taking antiretroviral medications. Most participants shared they would recommend the program to a friend. Thus, the results and positive reception suggest that NYC’s program could feasibly distribute HIV self-testing kits to and promote HIV testing among a diverse, high-risk MTSM population.
Smartphone mHealth app interventions with HIV self-testing kit distribution
Three smartphone apps have been designed and tested to encourage HIV prevention and bolster free HIV self-testing kit distribution among high-risk populations. A recently studied one is the HealthMindr app, which is based on the social cognitive theory. The app provides tailored HIV prevention plans and promotes preventive behaviors (e.g., distributing free HIV self-testing kits, reminding participants to regularly test for HIV, encouraging safe sex practices). From May through August 2015, Sullivan et al. used Facebook and other social networking sites to recruit 121 young MSM in Atlanta and Seattle. They received access to the HealthMindr app for four months. During the study period, 154 HIV self-testing kits were ordered with 53% (64/121) of participants ordering at least one kit. Approximately 81% (98/121) of participants completed a post-study evaluation. In the follow-up survey, 68% (34/50) of people who ordered HIV self-testing kits reported using the kits on themselves, and three participants had positive HIV test results. Furthermore, a majority rated the mobile app favorably (21).
Currently, two recently developed mobile applications are under evaluation. Biello et al. is spearheading a pilot randomized clinical trial to determine the usability and feasibility of the MyChoices mobile app in strengthening HIV prevention services among young MSM (22). By adapting the HealthMindr app and including relevant quizzes, videos, and infographics, the MyChoices app hopes to directly appeal to young MSM. The app incorporates the social cognitive theory of goal setting (e.g., plan to regularly test for HIV), self-efficacy (e.g., accomplish this goal by ordering free HIV self-testing kits through the app), and self-regulation (e.g., track and complete HIV self-testing) framework to modify behavior (e.g., obtain routine HIV testing). Since October 2018, participants from the University of North Carolina and Emory Center for Innovative Technology’s (iTech) clinical research locations in Boston, Massachusetts and Bronx, New York have been recruited from various venues, including social media platforms (e.g., Facebook, Grindr) frequently visited by young MSM.
Another iTech project is the LYNX mobile app, which is similar to its MyChoices counterpart but follows the information-motivation-behavioral skills model (23). Among its various functions, LYNX offers an electronic diary to record sexual behaviors (information), provides regular HIV testing reminders (motivation/behavioral skills), and offers delivery of free HIV self-testing kits (behavioral skills). Those with positive results can reach an on-call clinician or use the app’s chat function to connect with LYNX staff for assistance. Participants who enter positive test results into the app will be contacted by the research team and provided appropriate follow up care. The recruitment process for the LYNX pilot study, which started in October 2018, is similar to that for the MyChoices app except participants for the former are recruited from clinical research sites in Chicago, Illinois, and Tampa, Florida. Furthermore, participants may also be recruited from clinics (e.g., provider referral, medical chart reviews).
Results from the ongoing MyChoices and LYNX mobile app studies are pending. If both apps prove to be acceptable and feasible in increasing HIV prevention behaviors among young MSM, they will be compared to each other in another study to determine the more effective app (22,23).
Interactive mHealth interventions to supplement HIV self-testing process
Four studies combined HIV self-testing with direct communication technologies to address concerns of privacy, stigma, and loss to follow-up (15,16).
In 2017, two studies published by Stephenson et al. promote HIV testing among same sex male couples (Project Nexus) and transgender and gender non-conforming youth (TY) (Project Moxie) (24,25). In both studies, participants were recruited from social media (e.g., Facebook, Instagram), dating apps (e.g., Grindr, Scruff), and popular online community organization sites (e.g., Transgender Alliance and Affirming Transgender Rights). For the two studies, intervention groups received mail-delivered HIV self-testing kits and video counseling during the testing process, while control groups only received HIV self-testing kits. Both Project Nexus and Moxie are still in progress, but at the time of protocol publication, Project Nexus has recruited 219 same sex male couples, and Project Moxie has 130 TY participants. For Project Nexus, there was lower adherence to the intervention condition (77% (85/111) of same sex male couples) compared with the control condition (88% (95/108) of same sex male couples) (24). Overall, 6% (26/438) of Project Nexus participants had a positive HIV self-test result and 73% (19/26) were connected to follow-up care (24). Among the 130 TY participants recruited in Project Moxie, there was lower compliance among the experimental group compared with the control group as only 12 participants in the experimental group adhered to the intervention compared to 58 participants in the control group (25). Further conclusions concerning the efficacy of Projects Nexus and Moxie are pending as the results of the projects are forthcoming.
To streamline and ensure timely post-HIV self-testing follow-up care, Wray et al. designed the eTEST system comprised of a smartphone app that detects the opening of HIV self-testing kits via Bluetooth low energy beacons (26). Once the kit is opened, the app will notify staff members to conduct follow-up calls within 24 hours. The eTEST system is highly effective in identifying HIV self-testing usage and is acceptable among MSM. In 2018, Wray et al. compared the efficacy of the eTEST system to a standard group (i.e., traditional HIV self-testing kits with no follow-up calls) and a control group (i.e., letters about local clinic-based HIV testing) (27). These various conditions were repeated in three-month intervals during the seven-month study period. Eligible MSM with Android or iOS smartphones were recruited from social media sites (e.g., Facebook, Instagram), online dating apps (e.g., Grindr, Scruff), and direct outreach (e.g., flyers). Participants were randomly assigned to each group. Individuals in groups that received HIV self-testing (i.e., eTEST and standard HIV self-testing conditions) reported at least one repeat testing during the study period, while only 72% of those in the control group did so. Interestingly, repeat testing at later intervals (3 and 6 months) did not significantly differ between eTEST and standard groups, suggesting follow-up calls encouraging future HIV testing did not noticeably increase repeat testing compared with regularly distributing HIV self-testing kits. Furthermore, those assigned to the standard HIV self-testing group were less likely to receive risk reduction counseling compared with their eTEST and control counterparts, proposing that timely follow-up referral calls or in-person counseling sessions are effective in linking MSM to appropriate counseling services. Given the potential success of the eTEST system in promoting regular HIV testing among MSM, the study team is currently conducting a clinical trial based on the model they developed (28). Future data will provide insight on the cost-effectiveness of the eTEST system.
Discussion
Our findings suggest that some mobile health (mHealth) interventions may promote the use of and increase access to HIV self-testing kits among high-risk populations in the United States. By reducing stigma and allowing testing in a private setting, individuals may have been empowered throughout the testing process. The rapid screening could increase people’s awareness of their HIV serostatus, allowing them to seek follow up care and inform discordant partners of potential HIV transmission risk (15). HIV self-testing may also raise concerns (e.g., necessity of confirmatory testing, inadequate follow-up care) (14-16). Despite those potential drawbacks, our findings suggest the use of mHealth may alleviate those issues, especially among vulnerable populations.
Although mHealth-associated HIV self-testing efforts may increase serostatus awareness, it is important to improve their implementation in order to maximize their benefits (Table 2). First, determining the most effective mHealth method to distribute HIV self-testing kits is crucial to efficiently reach high-risk populations. Currently, our team is partnering with Dartmouth College to investigate the relative effectiveness of social media sites (e.g., Facebook), dating apps (e.g., Grindr), and informational websites (e.g., Google) in promoting HIV self-testing (29). Enrollment begins in August 2019, and the study anticipates recruiting 400 study subjects from eight geographically diverse regions. After clicking on advertisements placed on the web-based platforms, eligible participants (i.e., young Black/African American or Latino MSM aged 18–30 years old) are invited to complete baseline surveys and order free HIV self-testing kits. They will be followed up at two weeks and 60 days post-baseline. The primary outcome is the monthly rate at which HIV self-testing kits are requested by specific web-based platforms. Secondary outcomes include pre-exposure prophylaxis (PrEP) uptake and the impact of substance use on HIV self-testing and PrEP uptake. It is our hope to determine the most effective mHealth platform to deliver HIV self-testing kits to those most in need.

Full table
Second, regularly distributing HIV self-testing kits may be more effective in increasing repeat HIV testing compared with encouraging HIV testing behaviors via follow-up calls (27). Since mHealth could potentially increase access to HIV self-testing among high-risk populations (17,18), federal agencies and local health departments should support its expansion to ensure those communities regularly receive such screening tools.
Third, reducing the cost of HIV self-testing kits may increase such testing efforts. Studies and interventions reviewed in this article bypassed this issue by offering free kits. Currently, an OraQuick test kit costs $40 on the manufacturer’s website, which could put it out of reach of vulnerable populations (30-33). A potential solution may be for state Departments of Public Health to create partner programs with OraSure to reduce the cost of the kits for specific communities.
Fourth, strengthening linkage to post-HIV self-testing care may be necessary to improve mHealth-associated HIV self-testing. Some cities have implemented plans to support HIV care. In 2017, New York City’s Plan to End the HIV/AIDS Epidemic initiative invested $23 million to increase HIV prevention and health care programming by extending City STD clinic hours, promoting HIV and sexual health services, and supporting community-based HIV prevention and care programs (34,35). Since mHealth-facilitated HIV self-testing practices (e.g., HIV self-testing video-counseling, eTEST system, MyChoices and LYNX apps) could substantially strengthen post-HIV self-testing care (22,23,26,27), they should be incorporated into such follow-up programs to ensure high-risk populations receive appropriate care.
Finally, fostering partnerships between mHealth research groups, community partners, and public health agencies could increase HIV prevention and treatment services. A collaborative opportunity involves the Ending the HIV Epidemic: A Plan for America initiative. Launched by the federal administration in 2019, the program aims to decrease new HIV infections by 75% in the next five years and by 90% in the next ten years (36). Increasing HIV testing in communities with high HIV burdens is one of the initiative’s major goals. Given the promising success of mHealth-associated HIV self-testing in bolstering HIV testing, such screening methods should be included in the initiative.
There are several limitations to our narrative review. First, we did not conduct a systematic review, so we may have excluded studies that could have affected our results. Second, we only focused on studies conducted in the United States, potentially limiting the scope and applicability of our findings. Third, the studies in our review may have overestimated the effectiveness of mHealth interventions because studies with positive results may be more likely to be published compared with ones showing negative findings. Fourth, many studies in our review had low follow-up data, thus limiting our understanding to HIV self-testing kit distribution rather than testing behaviors and potentially overestimating the impact of the interventions. Finally, our review did not include studies focused on the cost-effectiveness of mHealth-associated HIV self-testing. Future research should delve further into this topic to better inform health policy geared towards promoting HIV self-testing.
Overall, studies included in our review suggest that implementing effective mHealth practices (e.g., combining regular mHealth distribution of eTEST HIV self-testing kits with well-supported follow-up facilities and staff) into public health programs could increase HIV testing and linkage to care among vulnerable communities, accelerating our efforts to prevent and control HIV infection in the United States.
Acknowledgments
We would like to acknowledge the UCLA Center for AIDS Research (National Institutes of Health grant P30AI028697), the Center for HIV Identification, Prevention, and Treatment Services (grant P30MH058107), and Team Klausner Saving Lives for their support. We also thank Elisha Ikhumhen for proofreading the article.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Centers for Disease Control and Prevention. HIV Surveillance Report. 2017;29.
- U.S. Department of Health and Human Services. National HIV/AIDS Strategy for the United States: Updated to 2020, 2015 Jul.
- Centers for Disease Control and Prevention. HIV Prevention Progress Report, 2019. 2019.
- Pant Pai N, Sharma J, Shivkumar S, et al. Supervised and unsupervised self-testing for HIV in high-and low-risk populations: a systematic review. PLoS Med 2013;10:e1001414. [Crossref] [PubMed]
- Spielberg F, Kurth A, Gorbach PM, et al. Moving from apprehension to action: HIV counseling and testing preferences in three at-risk populations. AIDS Educ Prev 2001;13:524-40. [Crossref] [PubMed]
- The World Health Organization and the Joint United Nations Programme on HIV/AIDS statement on HIV testing services: new opportunities and ongoing challenges. 2017.
- Zhang C, Li X, Brecht ML, et al. Can self-testing increase HIV testing among men who have sex with men: A systematic review and meta-analysis. PLoS One 2017;12:e0188890. [Crossref] [PubMed]
- Krause J, Subklew-Sehume F, Kenyon C, et al. Acceptability of HIV self-testing: a systematic literature review. BMC Public Health 2013;13:735. [Crossref] [PubMed]
- Centers for Disease Control and Prevention. Home Tests. 2019 Feb 22. Available online: https://www.cdc.gov/hiv/testing/hometests.html. Accessed Jun 3 2019.
- United States Food and Drug Administration. First Rapid Home-Use HIV Kit Approved for Self-Testing. Available online: https://www.fda.gov/consumers/consumer-updates/first-rapid-home-use-hiv-kit-approved-self-testing. Accessed Jun 3 2019.
- Roehr B. FDA approves “instant” HIV home test. BMJ 2012;345:e4636. [Crossref] [PubMed]
- The World Health Organization. Guidelines on HIV self-testing and partner notification: supplement to consolidated guidelines on HIV testing services. 2016 Dec.
- The World Health Organization. mHealth: Use of appropriate digital technologies for public health. 2018 Mar 26.
- LeGrand S, Muessig KE, Horvath KJ, et al. Using Technology to Support HIV Self-Testing Among Men Who Have Sex with Men. Curr Opin HIV AIDS 2017;12:425-31. [Crossref] [PubMed]
- Wood BR, Ballenger C, Stekler JD. Arguments for and against HIV self-testing. HIV AIDS (Auckl) 2014;6:117-26. [PubMed]
- Figueroa C, Johnson C, Verster A, et al. Attitudes and acceptability on HIV self-testing among key populations: a literature review. AIDS Behav 2015;19:1949-65. [Crossref] [PubMed]
- Huang E, Marlin RW, Young SD, et al. Using Grindr, a smartphone social-networking application, to increase HIV self-testing among Black and Latino men who have sex with men in Los Angeles, 2014. AIDS Educ Prev 2016;28:341-50. [Crossref] [PubMed]
- Rosengren AL, Huang E, Daniels J, et al. Feasibility of using Grindr™ to distribute HIV self-test kits to men who have sex with men in Los Angeles, California. Sex Health 2016;13:389-92. [Crossref] [PubMed]
- Virginia Department of Health. Using In-Home HV Testing and Online Screening to Increase Testing Uptake. National Alliance of State and Territorial AIDS Directors (NASTAD) Chair's Challenge. 2015. Available online: https://www.nastad.org/sites/default/files/HD_Success_Stories/virginia-home-hiv-testing.pdf. Accessed Jun 3 2019.
- Edelstein Z, Salcuni P, Khawja A, et al. Feasibility and Reach of a HIV Self-Test (HIVST) Giveaway, New York City, 2015-2016. CROI; Seattle, Washington 2017 Feb 13-16.
- Sullivan PS, Driggers R, Stekler JD, et al. Usability and acceptability of mobile comprehensive HIV prevention app for MSM. JMIR Mhealth Uhealth 2017;5:e26. [Crossref] [PubMed]
- Biello KB, Marrow E, Mimiaga MJ, et al. A mobile-based app (MyChoices) to increase uptake of HIV testing and pre-exposure prophylaxis by young men who have sex with men: protocol for a pilot randomized controlled trial. JMIR Res Protoc 2019;8:e10694. [Crossref] [PubMed]
- Liu A, Coleman K, Bojan K, et al. Developing a mobile app (LYNX) to support linkage to HIV/STI testing and PrEP for YMSM. JMIR Res Protoc 2019;8:e10659. [Crossref] [PubMed]
- Stephenson R, Freeland R, Sullivan SP, et al. Home-based HIV testing and counseling for male couples (Project Nexus): a protocol for a randomized controlled trial. JMIR Res Protoc 2017;6:e101. [Crossref] [PubMed]
- Stephenson R, Metheny N, Sharma A, et al. Providing home-based HIV testing and counseling for transgender youth (Project Moxie): protocol for a pilot randomized controlled trial. JMIR Res Protoc 2017;6:e237. [Crossref] [PubMed]
- Wray T, Chan PA, Simpanen E, et al. eTEST: developing a smart home HIV testing kit that enables active, real-time follow-up and referral after testing. JMIR Mhealth Uhealth 2017;5:e62. [Crossref] [PubMed]
- Wray TB, Chan PA, Simpanen E, et al. A pilot, randomized controlled trial of HIV self-testing and real-time post-test counseling/referral on screening and preventative care among men who have sex with men. AIDS Patient Care STDS 2018;32:360-7. [Crossref] [PubMed]
- The U.S. National Library of Medicine: ClinicalTrials.gov. eTest: Real-time, Remote Monitoring System for Home-based HIV Testing Among High-risk Men Who Have Sex with Men (eTest). Available online: https://clinicaltrials.gov/ct2/show/NCT03654690?term=etest&rank=3. Accessed Jul 17 2019.
- The National Drug Abuse Treatment's Clinical Trials Network: Northeast Node Active Research Studies. Using Social Media to Deliver HIV Self-Testing Kits and Link to Online PrEP Services. Available online: http://www.ctnnortheastnode.org/research/. Accessed Jul 10, 2019.
- Ibitoye M, Frasca T, Giguere R, et al. Home testing past, present and future: lessons learned and implications for HIV home tests. AIDS Behav 2014;18:933-49. [Crossref] [PubMed]
- Ng OT, Tan MT. HIV self-testing: money matters. Clin Infect Dis 2013;57:771-2. [Crossref] [PubMed]
- Orasure Technologies, Inc. OraQuick® In-Home HIV Test. Available online: https://shop.oraquick.com. Accessed Jun 5 2019.
- Walensky RP, Paltiel AD. Rapid HIV testing at home: does it solve a problem or create one? Ann Intern Med 2006;145:459-62. [Crossref] [PubMed]
- NYC Health. New HIV Outreach Effort Distributes Over 1,700 Free HIV Self-Test Kits To New Yorkers, Identifies High Number Of Untested New Yorkers. 2016 Nov 2.
- New York City Department of Health and Mental Hygiene. Ending the Epidemic (EtE) Overview. 2017 Jan 31. Available online: https://www1.nyc.gov/assets/doh/downloads/pdf/ah/ete-strategy.pdf. Accessed Jun 5, 2019.
- Azar A. Ending the HIV Epidemic: A Plan for America. U.S. Department of Health and Human Services. 2019 Feb 5. Available online: https://www.hhs.gov/blog/2019/02/05/ending-the-hiv-epidemic-a-plan-for-america.html. Accessed Jun 5 2019.
Cite this article as: Ko JS, Stafylis C, Klausner JD. Mobile health promotion of human immunodeficiency virus self-testing in the United States. mHealth 2020;6:10.


