The feasibility and acceptability of mobile health monitoring for real-time assessment of traumatic injury outcomes
Introduction
Injuries and their long-term consequences come at great cost to individuals, health systems and society at large. Each year more than 2.5 million people are hospitalized for an injury in the United States (US) (1). Over the last thirty years innovations in clinical practice and the implementation of accreditation standards for specialized trauma centers has yielded significant reductions in injury mortality (2-4). Now, approximately 95% of patients admitted to hospitals for trauma will survive to discharge (5,6). While a this represents a critical achievement for emergency and acute healthcare, there have not been comparable successes in the prevention of long-term dysfunction and decline in physical, mental, and social health after injury (7).
The organization and scope of trauma care can present important challenges to addressing comprehensive long-term and chronic patient needs. Trauma care services are often siloed within physically and disciplinarily discrete emergency, acute, and rehabilitation care settings (8). These divisions can limit a comprehensive continuum of care and may also obscure the true burden of long-term sequelae and need for enhanced post-hospitalization interventions.
Patients themselves are often not in a position to seek out the healthcare and supportive services that could improve the course of their recovery trajectory. For example, recent research on outcomes in predominately low income seriously injured Black men indicated the concurrence of tremendous psychological symptom burden after injury (9), and prohibitive barriers to mental health services ranging from financial constraints to a lack of knowledge of local mental health services (10). Enhanced screening at post-hospitalization outpatient follow-up may similarly miss patients most in need. Outpatient follow-up after trauma has an adherence rate as low as 50% (11), and is notably lower in patients lacking health insurance or enrolled in Medicaid (12).
With these constraints in mind, addressing comprehensive recovery in the community-setting and after injured people return home and attempt to return to their daily lives could yield substantial improvements in long-term outcomes (13,14). To date, however, there has been limited research that has systematically explored the breadth and temporal range of long-term injury sequelae. We do know that physical and psychological symptoms, as well as the social and economic challenges that commonly occur after injury are complex; current evidence suggests that functional and psychological symptoms and correlates of recovery, like return to work, often inter-relate as they appear and continue for months and years after hospitalization (13-20). Recovery outcomes are also impacted by the context of individual diversities in physiology, health status, lifestyles, and environmental exposures. Efforts are underway to identify key patients-reported outcomes in the year after hospitalization for trauma (21,22), which may assist in the prediction of outcomes most amenable to intervention. An emerging body of research is also strengthening the evidentiary basis for enhanced follow-up after hospitalization and the need better coordinate post-discharge care (23).
Additional interventions are needed to more fully address the complex and changing symptoms that injured people experience after hospitalization (24,25) and in a way that is responsive to variations in the physical and social environment in which people attempt to heal (26). As we consider the impact of social environments specifically, mobile technologies like smartphones are an increasingly integral aspect of most people’s daily lives. Although mobile technology-based prevention and treatment interventions have been used to monitor and transform outcomes across a myriad of health conditions, including long-term psychological symptomology (27-30), mobile health application for monitoring and addressing interrelated psychological, functional, and social outcomes in long-term traumatic injury recovery has not been unexplored.
The goal of this research was to identify the feasibility and acceptability of long-term mobile health monitoring in a population of patients who both experienced a serious traumatic injury and who live with existing barriers to health and social care after injury. The specific aims of were to: (I) elicit the perspectives of patients who had experienced a serious traumatic injury in the past 24 months on the acceptability, delivery, and content of a technology-assisted monitoring intervention, and (II) assess the feasibility of collecting indicators of recovery using both automated surveys delivered to patients’ smartphones and supplementary biometric data from commercially available wrist-worn fitness monitors. We hypothesized that technology-assisted monitoring would be an feasible and acceptable way to assess symptom burden, study patient-identified recovery priorities, and collect select biometric data markers of functional and psychological recovery. This pilot research is a first step in identifying the utility and implementation specifications of real-time monitoring of long-term physical, psychological and social outcomes in trauma patients using mobile technology. We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/mhealth-19-200).
Methods
Study design
This mixed- methods study was conducted in three phases: (I) qualitative interviews and development of the pilot monitoring platform; (II) a 3-month feasibility trial of mobile monitoring of patient-reported outcomes and biometric data; (III) post-implementation qualitative interviews. To achieve the aims of this study we leveraged an existing web-based health research platform called Way to Health (WTH) to deliver self-monitoring assessments, identify how patients prioritize their health and recovery needs, and to test the feasibility of collecting biometric data using a commercially available wrist-worn fitness monitor. WTH has demonstrated feasibility, acceptability, and utility with patients across the age spectrum and a variety of other chronic health conditions (31,32). Housed at the University of Pennsylvania, WTH has infrastructure and processes that emphasize data security and the privacy of health information collected through surveys and device integration. The study protocol was approved by the Institutional Review Board of the University of Pennsylvania.
Setting and participants
Access to patients with known barriers to health and social care after hospitalization for injury can be a challenge, but we were able to re-recruit participants from a recently concluded study of psychological outcomes after hospitalization in a cohort of over 600 predominately low-income Black men living in and near Philadelphia, Pennsylvania. This cohort study consecutively enrolled men 18 years or older, who self-identified as Black, resided in the Philadelphia Region and presented to a Level 1 Trauma Center and were subsequently hospitalized for care of a serious traumatic injury of any cause. The intent of this cohort study was to determine predictors of depression and PTSD at 3 months after injury. Men with diagnosed cognitive dysfunction or psychotic disorder, hospitalized for attempted suicide, or in current treatment for depression or PTSD were excluded from cohort eligibility (9). The cohort study had concluded approximately eight months prior to the initiation of this study, but informed consent included an assent to re-contact for future research.
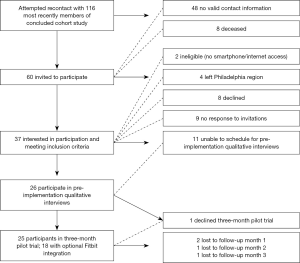
Our goal was to recruit a subsample of the cohort who had experienced an injury in the past 24 months. We began by recruiting the most recently injured cohort members for qualitative interviews and continued recruitment until we reached a 25-person sample for the mobile monitoring component of the pilot. Recruitment activities entailed contacting approximately 20% (n=116) of the original cohort through phone calls and letters and increasing our time frame to those injured in the past 36 months. Consecutive recruitment, beginning with the most recently injured members of the cohort, was used as a strategy to reduce biases related to recall and selection based on type or mechanism of injury. Of the original cohort, 50% lacked accurate or current contact information and over 5% had died based on records of the healthcare system in which they were initially treated for injury. Figure 1 illustrates the recruitment and retention scheme of this study.
Procedures, variables, and data sources
Data collection began with focus group and individual interviews. Six focus group interviews were conducted from June 2018 to August of 2018 in a community-based setting with groups of 3–8 participants. Five additional participants were interviewed individually to meet the needs of their work schedules or mobility limitations outside of the home (from, for example, severe neuropathies and paraplegia). All interviews were audio-recorded and conducted by the primary investigator who has experience in focus group and individual interview facilitation, using a semi-structured interview guide. Participants were prompted to describe residual symptoms they identified as related to their injury, perceptions of changes in their recovery needs since hospitalization, and facilitators and barriers that they felt were most influential in their recovery process. We also asked if and why they would they accept an intervention aimed to maintain longer term contact with health and social care providers, the technological specifications of mobile monitoring, and their perceptions of wrist-worn fitness monitors to track data like physical activity and sleep. Interviews also incorporated an opportunity to elicit initial feedback on the mobile interface, called Way to Recovery (WTR), designed for this pilot trial and concluded with an opportunity to initiate participants’ user profiles and establish a first connection to WTR. Participants were provided $50 in compensation for participation in these interviews. Twenty-five of 26 participants agreed to participate in a subsequent 90-day feasibility and acceptability trial.
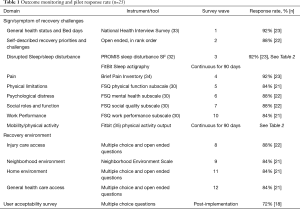
We then adapted the WTH platform for the WTR study to automate survey data collection through both email and text messages (SMS). WTR was programmed to deliver 12 surveys, at approximately one week apart, throughout the 90-day pilot trial. Each survey was available to the respondents for 48 hours. Each survey (see Table 1) comprised a unique aspect of trauma recovery (i.e., sleep quality, pain, mental health, physical activity). Survey response was monitored, and each individual with non-response was reminded up to two times via an email and text message reminder.
Full table
Full table
WTR assessed different patient outcomes at each wave of survey administration using items from survey instruments that have demonstrated validity and reliably for use with injured people and diverse patient populations. Prior research has identified a suite of concerns that injured people have articulated are important for their post-hospitalization recovery, including: achieving independence, re-establishing physical and mental wellbeing, resuming family roles and returning to work (34). To address these priority areas survey items were derived from the Functional Status Questionnaire (FSQ) to assess functional recovery in the domains of physical function, mental health, work performance, level of social interaction, and quality of social interaction (36). Sleep disturbance has been shown to predict poorer recovery during post-acute rehabilitation (37). We therefore also surveyed participants using the Patient-Reported Outcomes Measurement Information System (PROMIS) Sleep Disturbance Short Form (33). We assessed pain using the Brief Pain Inventory (38). We examined perceptions of environmental exposures during recovery using the Neighborhood Environment Scale (39), and questions about home environment and safety from the Ontario Rapid Risk Factor Surveillance System (40). In addition, we asked participants to recall the number of days in the past month spent in bed due to health as a measure of their residual disability [from the National Health Interview Survey (41)], and to answer open ended questions to: rank their most important recovery priorities, rank their most difficult recovery challenges, and identify where they would seek care if they experienced an injury-related health problem. At the end of the pilot trial, we also prompted participants to quantify, using a series of Likert scaled items, their perception of the acceptability and content of the WTR platform. Participants were given a $100 incentive at the end of each of the three months in which they completed automated surveys.
Eighteen of 25 participants met criteria (access to a smart phone and willingness to wear a wrist worn monitor 24 hours a day) and opted to pilot the integration of biometric data collection using a commercially available wearable monitor (Fitbit Charge 2) (42,43). Initiating biometric monitoring required meeting participants at their homes to instruct them on the set-up and maintenance of their device and its connection to WTR. Prior to the start of the 90-day data collection period, we collected Fitbit data for a minimum of 14 days to affirm data capture and troubleshoot technical difficulties participants encountered in using their device. Participants were prompted through emails and text messages on a weekly basis to synchronize their device to their smartphone to enable upload of data to the WTR platform. WTR collected data on daily step count (as a marker of physical activity), hour of initiation of sleep, sleep duration, sleep restlessness, resting heart rate and average hourly heart rate.
In April of 2019, after all participants had completed the pilot trial, two focus group interviews were conducted in a community-based setting with 8 participants. Five additional participants were interviewed from May through June of 2019 individually to accommodate their work schedules or mobility limitations outside of the home. All interviews were audio-recorded and conducted using a semi-structured interview guide. Participants were prompted to describe: their perceptions of their recovery since the last interview, the acceptability of WTR monitoring platform, delivery, content, and when applicable the acceptability and utility of Fitbit integration. Participants were provided $50 compensation for participation.
Analysis
Focus group and individual interview data were analyzed for thematic content using NVivo version 12 software for qualitative data management. These data were open coded iteratively by the PI and two research assistants and developed into a codebook that summarized overarching categories and themes. Additional focused content, such as specific reference to the acceptability of the WTR pilot was coded deductively. A third research assistant recoded the qualitative interviews using the final codebook and any inconsistencies or the emergence of new, previously uncoded content, were re-evaluated and discussed as a research team until consensus was achieved.
Data from quantitative and qualitative survey items were downloaded from the WTR platform. We descriptively analyzed survey data including calculation of summary statistics with means, medians, and ranges for continuous variables and frequencies for discrete variables in SPSS version 23.
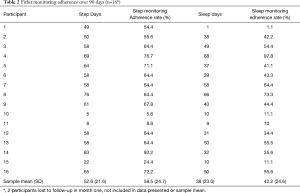
Data from the Fitbit monitors for the subsample who opted into this additional component of the pilot were also downloaded from the WTR platform. Fitbit monitoring adherence was assessed by examination of daily step count and sleep data for each subject. Days in which a subject recorded a non-zero step count was counted as a day that the subject actually wore the Fitbit. Days with zero steps were counted as non-adherence days, based on the assumption that the Fitbit was stationary, and thus not worn. Adherence data were converted into a binary variable to create a total number of adherence days for the 90-day monitoring period. Adherence percentage was calculated by taking this total of "adherence days" and using the 90-day monitoring period as the denominator. For sleep adherence, non-adherence days were counted as days with zero recorded sleep minutes, and adherence days were counted as days with any recorded sleep minutes. If there were no recorded sleep minutes, the subject was assumed to have not worn the Fitbit during sleep for that day. Days of sleep adherence were then divided by the 90-day monitoring period to calculate the sleep adherence percentage.
Results
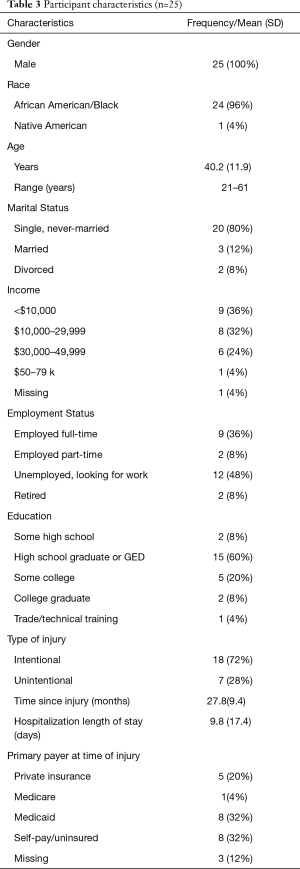
Table 3 describes participants’ demographic and injury characteristics. This sample included predominantly single, high-school educated Black men, across the adult age spectrum, with relatively low annual income, and more than half of whom had been injured intentionally (gun and other forms of interpersonal violence). Analysis of data from pre-implementation focus groups indicated that the majority of participants used their smartphones as a primary means of communication with their social network and to access the internet. All endorsed the perception of the potential feasibility and acceptability of responding to long-term symptom assessments via their smartphones. The constraint that was discussed by some was financial barriers specific to the cost of maintaining consistent cellular data services or replacing inoperable telephones.
Full table
Findings from the 90-day pilot trial of mobile monitoring using the WTR platform indicated participants’ preference for text-delivered communication and survey elicitation. Response rates for each wave of automated survey administration are illustrated in Table 2. The response rate was generally high (87%), ranging from 84–92% on each of the 12 individual assessments, though participation waned over the course of the 90-day pilot trial. In post-implementation interviews, participants remarked that some survey items, particularly those about environmental exposures felt repetitive or incongruent with their perception of recovery. At the end of 90-days, 4 participants had been lost to follow up, 2 who had opted for Fitbit monitoring. Two of participants were lost to follow up in month 1 (one for unknown reasons/unable to recontact, one for acute hospitalization), a third in month two due to need for inpatient psychiatric care, and a final participant in month three for unknown reasons.
After loss to follow-up there were 16 participants with Fitbit data. Adherence to Fitbit monitoring, illustrated in Table 2, was generally lower than 50%, but ranged widely indicating some very low adherence and some very high adherence. Adherence rates also differed by daytime and nighttime monitoring periods, suggesting that some participants who were willing to wear a wrist-worn fitness monitoring during daytime activities were not willing to do so during night time activities and periods of sleep.
Step data, as a marker for physical activity, ranged from 277 to 12,466 steps per day (representing approximately a quarter mile to 5 miles of walking per day). The Fitbit also measured each participant’s average daily resting heart rate and then projected daily activity intensity based on time spent at ranges above the resting heart rate. These are measured as total daily minutes “out of range” of the resting heart rate, total minutes in “fat burn mode”, total minutes in “cardio” mode, and total minutes in “peak” mode. The average resting heart rate across participants was 69 beats per minute (SD =6.6) and ranged from 56.8 to 79.3 beats per minute. The time spent out of “rest” for heart rate indicated at least an hour of activity per day for all participants with Fitbit data. Some never entered the “cardio” or “peak” ranges of heart rate, which might indicate that those participants who were not engaged in regular physical activity or exercise. This may or may not be due to their limitations post-injury.
The minimum sleep level, as an average per participant, was less than two hours per sleep cycle. The high end of the range for all participants was 447.8 minutes, which is just under 7 and a half hours of sleep. The average duration of sleep across participants was approximately 5 hours. Several participants had periods of daytime sleeping and periods of wakefulness during nighttime hours, which may reflect daytime sleepiness, sleep disturbance, and/or evening and nighttime activity and employment. Although there are known limitations to the accuracy of these data when compared to clinical sleep studies and research-grade polysomnography devices (43), as a basic indicator of sleep difficulties or disturbances in long-term injury recovery this data may have use in conjunction with patient-reported outcomes. For example, we collected self-reported sleep evaluations using the PROMIS sleep disturbance short form and evaluated the pilot sample’s risk scores (Table 4). In concert with the limited sleep durations indicated by sleep cycle output from sub-sample who adhered to Fitbit monitoring, over 25% of participants reported mild sleep disturbance, and nearly 50% reported moderate to severe disturbance.

Full table
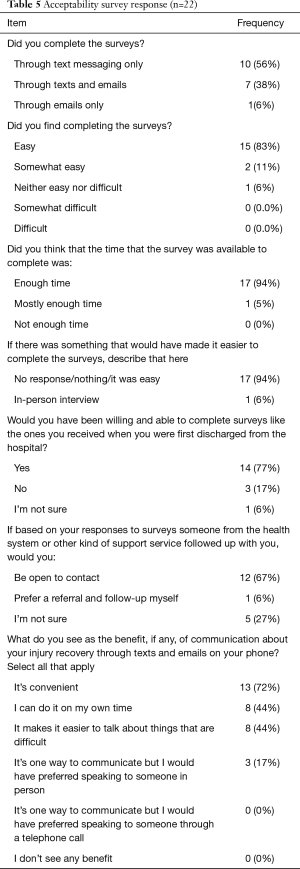
The acceptability of mobile elicitation of patient-reported outcomes and wrist-worn biometric monitors was assessed through a mobile survey and post-implementation interviews. The results of the survey are presented in Table 5. Overall, the participants found mobile monitoring to be acceptable, highlighting the benefit of convenience and the flexibility to reflect and respond to survey items at their own pace. Participants also offered additional feedback on the perceived benefit of long-term engagement with health and social services after hospitalization and the opportunity for enhanced peer-group interactions. These findings were reinforced in post-implementation interviews which highlighted, in addition to the acceptability of mobile monitoring, the value of self-monitoring through use of the Fitbit device to track physical activity and sleep on its smartphone application. The major themes and exemplar quotes from analyses of these data are outlined in Table 6. The one disadvantage cited about mobile monitoring concerned information privacy and control over who would be able to surveil physical activity and sleep data.
Full table

Full table
An unanticipated finding from this study was the extent to which it established the feasibility of collecting qualitative assessments of long-term injury outcomes and patient experience through text-delivered mobile elicitation. One example is that participants were asked in an open-ended item to identify their top three priorities when recovering from injury and rank order these priorities from most important to least important. Twenty-two participants responded to this question. Of these participants, 3 responded “none.” The most common theme identified for “Priority 1” highlighted physical wellness and restoration. Responses included relief from physical pain, “getting better,” “back to normal” or questions about the extent to which they would return to pre-injury levels of function as in: “Will I walk the same way as before my injury?” The second most common priority was related to financial security and employment, as in: “how was I going to be able to provide a living for myself with no savings and no income.” Additional themes identified as priorities concerned mental health, family stress and safety. Five respondents described mental health as a particular priority in terms of symptom management, for example: “dealing with depression”, “try and get over my anxiety”, and “becoming happy again”. Five respondents identified family stress as a priority describing concern for the impact of their injury within their family units, households, and social network, as in, “how this injury impacts my children and the rest of my loved ones”.
Discussion
As hypothesized, we found that overall, mobile health monitoring was a feasible and acceptable way to assess long-term symptom burden, examine patient-reported recovery outcomes and collect select biometric indicators in a sample of patients with past and/or current barriers to health and social care resources. Of perhaps equal importance to these findings, is the extent to which the research process demonstrated the healthcare disenfranchisement and vulnerability of the patient cohort from which we recruited this pilot sub-sample. Within 12 months of hospitalization, and in approximately 20% of a cohort of over 600 seriously injured Black men hospitalized at trauma center in Philadelphia, half lacked accurate or current contact information and 5% had died based on records of the healthcare system in which they were initially treated for injury. This highlights the urgent need for interventions that initiate and extend a continuity of long-term engagement with tools like mobile health monitoring. Similar to the limited corpus of research on long-term outcomes after trauma, we found that many patients in this pilot study continued to express signs and symptoms of poor recovery and chronic injury sequalae months and years after their hospitalization (13,15,44-47).
Wearable device data may have multiple benefits for understanding and improving long-term injury recovery. As demonstrated here, these kind of technologies can be a mechanism through which to collect and integrate data like step count and hours of sleep with patient-reported ratings of their functional status and sleep. These biometric data may offer an additional way to assess the emergence of sleep disrupting disorders like PTSD after a traumatic event (48), or the impact that psychological symptoms like traumatic stress or depression interplay with other indicators of recovery like mobility outside of the home and undisturbed sleep. Wearable device data may also be a way to monitor for real-time indicators that patients are in need of enhanced outreach and services, if for example, there are notable reductions or changes in patterns of biometric data output (49). Finally, as expressed by participants in this study, self-monitoring of data may offer motivation and validation to individuals in their own attempts to regain or improve physical mobility, strength, and sleep hygiene (35) in the aftermath of an injury.
These findings must be interpreted in the context of this study’s limitations. First and foremost, this is pilot research that did not intend or achieve, by design, a sample size sufficient to demonstrate any statistically significant associations between patient characteristics and long-term outcomes. We limited recruitment to a patient population of Black men residing in or near Philadelphia which may not be representative of the many other patients and socio-ecologic contexts associated with risks for poor long-term recovery trajectories after injury. The participants that we were able to recruit had a relatively wide range of time since injury (12–36 months) which could impact their reactions to, and responses to mobile monitoring using surveys and Fitbit monitors. We were also unable to assess the feasibility, acceptability, and utility of WTR initiated at or closer to the time of hospital discharge. The biometric data we collected on step count, sleep, and heart rate were through a commercially available wearable monitor, which has known limitations in its accuracy and reliability when compared to research-grade devices (42,43,49-51). Finally, we had a moderate loss to follow-up (16%) and waning response rate to automated survey elicitation over the 90-day trial.
Despite these limitations, to our knowledge, this is the first trial of mobile monitoring for collective long-term physical, psychological and social outcomes after injury. We were able to demonstrate acceptability in an often underserved patient population by engaging community-based recruitment activities and participant interviews. In addition, we demonstrated the feasibility of automated data collection of patient-reported outcomes and the utility a text (SMS) interface for eliciting qualitative outcome assessments long after the injuring event and hospitalization.
This study adds evidence to support efforts to more systematically and comprehensively conceptualize the aftermath of physical trauma as an often long-term and chronic health condition (7). Previous research has demonstrated the feasibility and utility of mobile health assessment and interventions for psychological trauma after physical injuries (27,30). This study extends these findings to a wider range of postinjury sequalae. It also prompts several opportunities for future research aimed to improve long-term injury outcomes and enhance the current continuum of trauma care services. Along with efforts to more systematically collect patient reported outcomes and monitor biometric indicators of recovery using wearable devices, implementation research is needed to investigate how clinical or social services would ideally integrate these data within practice environments and to establish its relative value over current clinical paradigms. There is also need to evaluate mobile monitoring interventions like WTR to optimize its feasibility and acceptability in a diverse patient populations, across different types of injury and levels of injury severity. Further research is also warranted to evaluate how to best balance the accuracy, effort and timeliness of patient-reported outcomes and biometric data collection. It may be that there are different benefits to using continuous (wearable), periodic (automated surveys) and repeated measurement of outcomes, or even ecologic momentary assessments (24) (deployed several times a day) to understand and improve common long-term outcomes like sleep, pain, mobility, mental health and quality of life.
Conclusions
This study identifies that mobile health technologies are a feasible and acceptable tool for monitoring and studying long-term injury sequalae in the community-setting. Moreover, it was shown to be acceptable in a cadre of trauma patients with known outcome disparities, access barriers, and a history of disenfranchisement from health and social care resources. Future research may leverage this novel strategy, refining its application to address current limitations in the reliability and accuracy of commercially available wearable sensors, relative benefits of different mobile data collection strategies, integration within current clinical paradigms and generalizability across injured populations and social environments.
Acknowledgments
We wish to acknowledge Daniel Holena, Nali Asamoah, K. Catalyst Twomey, Christie Delgadillo and Abeselom Gebreyesus for their contributions.
Funding: This work was supported by the Rita and Alex Hillman Foundation and the University of Pennsylvania University Research Foundation (PI: Jacoby). Research reported in this publication was also supported by the National Institute of Nursing Research of the National Institutes of Health under Award Number R01NR013503 (PI: Richmond). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Mei R Fu) for the series “Real-Time Detection and Management of Chronic Illnesses” published in mHealth. The article was sent for external peer review organized by the Guest Editor and the editorial office.
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/mhealth-19-200
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/mhealth-19-200). The series “Real-Time Detection and Management of Chronic Illnesses” was commissioned by the editorial office without any funding or sponsorship. SFJ reports grants from Alex & Rita Hillman Foundation and the University of Pennsylvania University Research Foundation, during the conduct of the study. AR reports grants from Alex & Rita Hillman Foundation and the University of Pennsylvania University Research Foundation, during the conduct of the study. JLW reports grants from Alex & Rita Hillman Foundation and the University of Pennsylvania University Research Foundation, during the conduct of the study. TSR reports grants from National Institutes of Health/NINR, during the conduct of the study. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional review board of the University of Pennsylvania (Protocol # 824456) and informed consent was obtained from all individual participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Centers for Disease Control and Prevention (CDC). Key injury and violence data 2017. Available online: https://www.cdc.gov/injury/wisqars/overview/key_data.html
- Glance LG, Osler TM, Mukamel DB, et al. Impact of trauma center designation on outcomes: Is there a difference between level I and level II trauma centers? J Am Coll Surg 2012;215:372-8. [Crossref] [PubMed]
- Haas B, Jurkovich GJ, Wang J, et al. Survival Advantage in Trauma Centers: Expeditious Intervention or Experience? J Am Coll Surg 2009;208:28-36. [Crossref] [PubMed]
- Garwe T, Cowan LD, Neas B, et al. Survival benefit of transfer to tertiary trauma centers for major trauma patients initially presenting to nontertiary trauma centers. Acad Emerg Med 2010;17:1223-32. [Crossref] [PubMed]
- Glance LG, Osler TM, Mukamel DB, et al. Outcomes of Adult Trauma Patients Admitted to Trauma Centers in Pennsylvania, 2000-2009. Arch Surg 2012;147:732. [Crossref] [PubMed]
- Davidson GH, Hamlat CA, Rivara FP, et al. Long-term Survival of Adult Trauma Patients. JAMA 2011;305:1001-7. [Crossref] [PubMed]
- Haider AH, Dankwa-Mullan I, Maragh-Bass AC, et al. Setting a National Agenda for Surgical Disparities Research: Recommendations From the National Institutes of Health and American College of Surgeons Summit. JAMA Surg 2016;151:554-63. [Crossref] [PubMed]
- Richmond TS, Aitken LM. A model to advance nursing science in trauma practice and injury outcomes research. J Adv Nurs 2011;67:2741-53. [Crossref] [PubMed]
- Richmond TS, Wiebe DJ, Reilly PM, et al. Contributors to Postinjury Mental Health in Urban Black Men With Serious Injuries. JAMA Surg 2019;154:836-43. [Crossref] [PubMed]
- Jacoby SF, Rich JA, Webster JL, et al. 'Sharing things with people that I don't even know': help-seeking for psychological symptoms in injured Black men in Philadelphia. Ethn Health 2020;25:777-95. [Crossref] [PubMed]
- Dalton MK, Fox NM, Porter JM, et al. Outpatient follow-up does not prevent emergency department utilization by trauma patients. J Surg Res 2017;218:92-8. [Crossref] [PubMed]
- Hansen L, Shaheen A, Crandall M. Outpatient follow-up after traumatic injury: Challenges and opportunities. J Emerg Trauma Shock 2014;7:256-60. [Crossref] [PubMed]
- Holbrook TL, Anderson JP, Sieber WJ, et al. Outcome after major trauma: 12-month and 18-month follow-up results from the Trauma Recovery Project. J Trauma 1999;46:765-71. [Crossref] [PubMed]
- Holbrook TL, Hoyt DB. The Impact of Major Trauma: Quality-of-Life Outcomes Are Worse in Women than in Men, Independent of Mechanism and Injury Severity. J Trauma 2004;56:284-90. [Crossref] [PubMed]
- Holbrook TL, Anderson JP, Sieber WJ, et al. Outcome after major trauma: discharge and 6-month follow-up results from the Trauma Recovery Project. J Trauma 1998;45:315-23; discussion 323-4. [Crossref] [PubMed]
- Richmond TS, Kauder D, Hinkle J, et al. Early predictors of long-term disability after injury. Am J Crit Care 2003;12:197-205. [Crossref] [PubMed]
- Richmond TS, Ruzek J, Ackerson T, et al. Predicting the future development of depression or PTSD after injury. Gen Hosp Psychiatry 2011;33:327-35. [Crossref] [PubMed]
- MacKenzie EJ, Cushing BM, Jurkovich GJ, et al. Physical impairment and functional outcomes six months after severe lower extremity fractures. J Trauma 1993;34:528-38. [Crossref] [PubMed]
- Zatzick DF, Rivara FP, Nathens AB, et al. A nationwide US study of post-traumatic stress after hospitalization for physical injury. Psychol Med 2007;37:1469-80. [Crossref] [PubMed]
- Zatzick DF, Rowhani-Rahbar A, Wang J, et al. The Cumulative Burden of Mental, Substance Use, and General Medical Disorders and Rehospitalization and Mortality After an Injury. Psychiatr Serv 2017;68:596-602. [Crossref] [PubMed]
- Rios-Diaz AJ, Herrera-Escobar JP, Lilley EJ, et al. Routine inclusion of long-term functional and patient-reported outcomes into trauma registries. J Trauma Acute Care Surg 2017;83:97-104. [Crossref] [PubMed]
- Haider AH, Herrera-Escobar JP, Al Rafai SS, et al. Factors Associated With Long-term Outcomes After Injury: Results of the Functional Outcomes and Recovery After Trauma Emergencies (FORTE) Multicenter Cohort Study. Ann Surg 2020;271:1165-73. [Crossref] [PubMed]
- Wegener ST, Carroll EA, Gary JL, et al. Trauma Collaborative Care Intervention: Effect on Surgeon Confidence in Managing Psychosocial Complications After Orthopaedic Trauma. J Orthop Trauma 2017;31:427-33. [Crossref] [PubMed]
- Heron KE, Smyth JM. Ecological momentary interventions: Incorporating mobile technology into psychosocial and health behaviour treatments. Br J Health Psychol 2010;15:1-39. [Crossref] [PubMed]
- Ramanathan N, Swendeman D, Comulada WS, et al. Identifying preferences for mobile health applications for self-monitoring and self-management: focus group findings from HIV-positive persons and young mothers. Int J Med Inform 2013;82:e38-46. [Crossref] [PubMed]
- Verbrugge LM, Jette AM. The disablement process. Soc Sci Med 1994;38:1-14. [Crossref] [PubMed]
- Price M, Van Stolk-Cooke K, Legrand AC, et al. Implementing assessments via mobile during the acute posttrauma period: feasibility, acceptability and strategies to improve response rates. Eur J Psychotraumatol 2018;9:1500822. [Crossref] [PubMed]
- Price M, Ruggiero KJ, Ferguson PL, et al. A feasibility pilot study on the use of text messages to track PTSD symptoms after a traumatic injury. Gen Hosp Psychiatry 2014;36:249-54. [Crossref] [PubMed]
- Davidson TM, Bunnell BE, Saunders BE, et al. Pilot Evaluation of a Tablet-Based Application to Improve Quality of Care in Child Mental Health Treatment. Behav Ther 2019;50:367-79. [Crossref] [PubMed]
- Anton MT, Ridings LE, Gavrilova Y, et al. Transitioning a technology-assisted stepped-care model for traumatic injury patients to a fully remote model in the age of COVID-19. Couns Psychol 2020. [Crossref]
- Mehta SJ, Troxel AB, Marcus N, et al. Participation Rates With Opt-out Enrollment in a Remote Monitoring Intervention for Patients With Myocardial Infarction. JAMA Cardiol 2016;1:847. [Crossref] [PubMed]
- Van Egeren LF, Sparrow AW. Ambulatory monitoring to assess real-life cardiovascular reactivity in Type A and Type B subjects. Psychosom Med 1990;52:297-306. [Crossref] [PubMed]
- Yu L, Buysse DJ, Germain A, et al. Development of Short Forms From the PROMISTM Sleep Disturbance and Sleep-Related Impairment Item Banks. Behav Sleep Med 2011;10:6-24. [Crossref] [PubMed]
- Aitken LM, Chaboyer W, Jeffrey C, et al. Indicators of injury recovery identified by patients, family members and clinicians. Injury 2016;47:2655-63. [Crossref] [PubMed]
- Chiauzzi E, Rodarte C, DasMahapatra P. Patient-centered activity monitoring in the self-management of chronic health conditions. BMC Med 2015;13:77. [Crossref] [PubMed]
- Cleary PD, Jette AM. Reliability and validity of the functional status questionnaire. Qual Life Res 2000;9:747-53. [Crossref]
- Alessi CA, Martin JL, Webber AP, et al. More daytime sleeping predicts less functional recovery among older people undergoing inpatient post-acute rehabilitation. Sleep 2008;31:1291-300. [PubMed]
- Tan G, Jensen MP, Thornby JI, et al. Validation of the Brief Pain Inventory for chronic nonmalignant pain. J Pain 2004;5:133-7. [Crossref] [PubMed]
- Crum RM, Lillie-Blanton M, Anthony JC. Neighborhood environment and opportunity to use cocaine and other drugs in late childhood and early adolescence. Drug Alcohol Depend 1996;43:155-61. [Crossref] [PubMed]
- Prince SA, Kristjansson EA, Russell K, et al. A multilevel analysis of neighbourhood built and social environments and adult self-reported physical activity and body mass index in Ottawa, Canada. Int J Environ Res Public Health 2011;8:3953-78. [Crossref] [PubMed]
- Verbrugge LM. The Disability Supplement to the 1994-1995 National Health Interview Survey. National Center for Health Statistics, 1995.
- Benedetto S, Caldato C, Bazzan E, et al. Assessment of the fitbit charge 2 for monitoring heart rate. PLoS One 2018;13:e0192691. [Crossref] [PubMed]
- de Zambotti M, Goldstone A, Claudatos S, et al. A validation study of Fitbit Charge 2™ compared with polysomnography in adults. Chronobiol Int 2018;35:465-76. [Crossref] [PubMed]
- Polinder S, Van Beeck EF, Essink-Bot ML, Toet H, Looman CWN, Mulder S, et al. Functional outcome at 2.5, 5, 9, and 24 months after injury in the Netherlands. J Trauma 2007;62:133-41. [Crossref] [PubMed]
- Jacoby SF, Shults J, Richmond TS. The effect of early psychological symptom severity on long-term functional recovery: A secondary analysis of data from a cohort study of minor injury patients. Int J Nurs Stud 2017;65:54-61. [Crossref] [PubMed]
- Velmahos CS, Herrera-Escobar JP, Al Rafai SS, et al. It still hurts! Persistent pain and use of pain medication one year after injury. Am J Surg 2019;218:864-8. [Crossref] [PubMed]
- Tan AL, Chiong Y, Nadkarni N, et al. Predictors of Change in Functional Outcome at six months and twelve months after Severe Injury: A Retrospective Cohort Study. World J Emerg Surg 2018;13:57. [Crossref] [PubMed]
- Milanak ME, Zuromski KL, Cero I, et al. Traumatic Event Exposure, Posttraumatic Stress Disorder, and Sleep Disturbances in a National Sample of U.S. Adults. J Trauma Stress 2019;32:14-22. [Crossref] [PubMed]
- Dias D, Paulo Silva Cunha J. Wearable Health Devices-Vital Sign Monitoring, Systems and Technologies. Sensors (Basel) 2018;18:2414. [Crossref] [PubMed]
- Case MA, Burwick HA, Volpp KG, et al. Accuracy of smartphone applications and wearable devices for tracking physical activity data. JAMA 2015;313:625-6. [Crossref] [PubMed]
- Feehan LM, Geldman J, Sayre EC, et al. Accuracy of Fitbit Devices: Systematic Review and Narrative Syntheses of Quantitative Data. JMIR Mhealth Uhealth 2018;6:e10527. [Crossref] [PubMed]
Cite this article as: Jacoby SF, Robinson AJ, Webster JL, Morrison CN, Richmond TS. The feasibility and acceptability of mobile health monitoring for real-time assessment of traumatic injury outcomes. mHealth 2021;7:5.