mHealth and big data will bring meaning and value to patient-reported outcomes
Mobile technology has changed how we interact with our families, friends, colleagues, and even our finances in an extremely personalized way. Isn’t it time it had the same impact on our health?
Early adopters of mHealth technology saw the promise even without significant research because the idea just made sense: our phones have become our life, even appendage-like, and managing our health is a key part of life, so managing your health on your phone the same way you can manage your schedule, your finances, and even your love life seems a natural progression of that. At the same time, skeptics criticized that health systems would never embrace mobile health, and that people who were interested in managing their health in a mobile manner skewed towards the young and healthy, and that these always-on devices were creating too many data points for anyone to make sense of.
These are valid criticisms that may have once been true, but the tide is changing. First, major health systems like Sentara, a not-for-profit 12 hospital system and integrated health plan, are adopting mobile health technologies from new companies like Wellpepper (the author’s company) (1). Organizations like Mayo Clinic and Partners Healthcare have their own connected health departments in which mobile is a major initiative, and many health systems have created their own venture funds to capitalize on these new technologies.
Second, mobile health crosses age and socio-economic boundaries. Pew Research shows that 84% of people with incomes of less than $30,000 per year have mobile phones, as does 74% of those 65 plus (2). In a study conducted with pre-diabetes Medicare Advantage participants with an average age of 70, Omada Health showed 85% adherence over 6 months (3). At Wellpepper, we saw similar results of 81% adherence over 3 months from a group of people 50–75 years old with Parkinson’s disease (4). At Wellpepper we have demonstrated over 70% patient engagement across our patient population, and people over 50 have higher levels of engagement than our system-wide average. In 2015, mobile health research really reached the masses when over 11,000 people downloaded an Apple Research Kit cardiovascular survey study in 1 week (5).
However, the last issue of “too many data points” remains true, and determining which data points have the most relevance, as well as making sense of and adding context to the tsunami of mobile health data, much of it coming from sensors and patient-reported outcomes is key to harnessing the power of mHealth.
In this article we will explore the promise and value of mHealth for collecting patient-reported outcomes, and how big-data can be used to personalize and improve care, disrupt the way we do health outcomes research, shorten the cycle between clinical research and clinical practice implementation, and aid in delivering personalized care.
Patient-reported outcomes
Tracking any type of patient outcomes was once thought to be heretical in the medical profession. In 1914, Dr. Ernest Codman, a pioneer in hospital reform and outcome management, lost privileges at Massachusetts General for suggesting that surgeon competence be evaluated based on outcomes (6). He was also an advocate that this surgeon evaluation and outcome data be made public.
Today, the shift to value-based payments is increasing the importance of tracking outcomes and patient-reported outcomes in particular have been given a major boost from Centers for Medicare and Medicaid Services (CMS) initiatives like the Comprehensive Care for Total Joint initiative: a value-based program that includes patient-reported outcomes as part of the payment and bonus criteria (7). Patient-reported outcome measures are those which represent information that can only be perceived by a patient; some examples include pain levels, general mood, and energy level. These measures represent the patient’s personal experience and are a key indicator of a successful outcome: if a patient doesn’t believe they’ve had a successful outcome, have they?
There is a great opportunity for mobile health to play a role in the evolution of patient-reported outcomes, and speed adoption in ways that were not previously possible, and while the number of data points collected may increase, the number of questions we need to ask patients to get to valid, useable, and actionable data can actually decrease.
How is this possible? Take a look at how we collect outcomes today: researchers at academic medical institutions create survey instruments. For these instruments to be considered valid they need to be completed by a certain number of patients over a long period of time. Paper surveys are distributed to patients, results are tabulated, and repeated at some interval. Validating a survey takes a lot of effort, and once a survey is considered validated, it is difficult to modify. As a result, these surveys are not adaptable to an individual patient experience. As well, as to not miss possible correlations and causations when analyzing data, these surveys tend to err by asking too many questions. The HCAHPS survey is a good example of this: a patient satisfaction survey that has 32 questions (8). Compare that to the standard satisfaction measure used in business: Net Promoter Score, which has one question: how likely are you to recommend this business/person/service?
Think back to the Apple Research Kit example mentioned earlier: if even 5% of the 11,000 people who signed up for the cardiovascular study continued to track results over time, those studies would have enough responses to be validated. If all patients continued, then there may be enough diversity in the population of respondents to do analysis on sub-sets and specific demographics, like left-handed ukulele playing 65 year-olds. Compare this to today when clinical trials are often not conducted on diverse populations even though we know that drugs can affect people of different genders and races differently (9).
Mobile health offers the ability for significantly more granular measures based on each patient and each patient’s experience rather than the gross measures of today’s standardized outcome surveys.
The patient experience
Today collection of these outcomes is most commonly done manually. What’s most striking about the current way these patient-reported surveys outcome are defined and collected is that data is not available in real-time to the clinician either to help with a diagnosis or track progress. Patients have a worse experience: they never see their results or scores, they cannot compare results over time, and they certainly can’t see how they are progressing against their peers or expected outcomes. Tracking outcomes on mobile devices solves all of these problems and provides a positive feedback loop for patients.
Comparing these standardized outcome reports collected via mobile device to other data points from passive data collection through integrated sensors on the mobile device can provide insight and further improve care. For example, for a patient who has had a total joint replacement, step count pre- and post-surgery is an interesting clinical outcome useful to both patients and providers indicating how that patient is recovering.
In an analog world, where collecting and analyzing data was expensive and time consuming the idea of tracking outcomes against a patient’s own goal was impossible. In a mobile world with personalized care plans and outcome measures, patients can manage their own progress by comparing their outcomes to their own personal goals and other clinical factors like pain management. The mobile device becomes not just a tool for retroactively collecting data, but rather an actionable and outcome-driven care plan that enables patients and providers to view and act on outcomes in real-time.
Now this is where big data and machine-learning need to enter the picture again, because while we all want completely personalized and real-time care, we need data processing economies of scale to make this happen. One of the promises of the real-time data collection and analysis possible with mHealth is the ability to shorten the 17-year cycle from research to clinical implementation (10).
Another benefit will be the ability to determine which outcome measures actually best represent a successful outcome: consider reducing the 32-question HCAHPS to the 1 question Net Promoter Score (11). It’s generally thought that in each standardized-outcome report there are one or two questions that truly represent a successful outcome. With enough data, the correlation between those questions and the clinical outcomes can be determined and we can decrease the number of survey questions. Or we can determine that those “success” questions are different for each patient and we can create care plans that are personalized to track both clinical and life outcome measures that are most important to each patient.
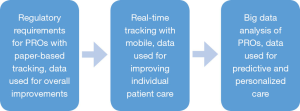
We can also use this data to adjust care and make recommendations to improve outcomes. If by analyzing the data, we notice that patients who answer a question in a certain way are more likely to have a particular outcome, then care interventions can be made in real time rather than retrospectively. Figure 1 depicts the evolution of patient-reported outcomes from a regulatory requirement to core care improvements.
In a world of real-time patient reported outcomes and sensor data, there may be too many data points coming in from patients for an individual clinician or even a health system to process and identify patterns, and this is where computing power really shines, and we can use it to scale the abilities of our care-givers as well as empower patients to manage their own care. At the same time, the deluge of data changes the way we process information to draw conclusions. In a seminal article in 2008, Wired predicted that the deluge of data will change the scientific method from hypothesis, model, and test (essentially how PRO surveys work today), to crunch the data and look for correlations (12).
In 2011, IBM announced that IBM Watson had learned as much as a 2nd year medical student (13). By 2013 it claimed to diagnose cancer better than physicians (14). The amount of medical information that physicians need to stay on top of is staggering; add to that the exponential growth in data and it’s overwhelming. However, big data doesn’t mean that computers take over care but that we use them to make sense of the data and enable humans to provide super-human and extremely personalized care.
Conclusions
Previously doctors needed to make decisions with only a few dozen bytes of information that they would glean from a visit, for example blood pressure, heart rate, height, weight, and the patient’s chief concern. The advent of mobile health enables the average person to passively throw off gigabytes of data through passive health monitors and kilobytes of clinically-relevant data through patient-reported outcomes, symptom tracking, adherence, and medication tracking. Without the ability to make sense of this data in the context of care and to enable both patients and care givers to glean actionable insight, this data is noise. Widespread mobile adoption and cloud computing when applied to healthcare will enable us to not only make sense of this information but to use it draw insights and shorten the cycle from research to clinical implementation. It will help healthcare providers and organizations scale with predictive suggestions based on each individual patient’s experience and the wealth of data collected from all patients.
Acknowledgements
The author thanks Ravi Komatireddy, MD, Assistant Professor, Scripps Translational Science Institute, co-founder Reflexion Health, and Mike Van Snellenberg, CTO and co-founder, Wellpepper, Inc.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Sentara healthcare chooses Wellpepper for mobile patient engagement in headache care. PRNewswire, Nov 2015. Available online: http://www.prnewswire.com/news-releases/sentara-healthcare-chooses-wellpepper-for-mobile-patient-engagement-in-headache-care-300181576.html
- Mobile Technology Fact Sheet. Pew Research. Available online: http://www.pewinternet.org/fact-sheets/mobile-technology-fact-sheet/
- Comstock J. Can 70-year olds lose weight with digital health? Omada, Humana data says yes. Mobihealth News, Nov 2015. Available online: http://mobihealthnews.com/48484/can-70-year-olds-lose-weight-with-digital-health-omada-humana-data-says-yes
- Rattey J. Take one app and call me in the morning. Inside Sargent, 2014. Available online: http://www.bu.edu/sargent/about-us/our-publications/inside-sargent-2014/rehab-revolution/
- Thousands have already signed up for apple’s research kit. Bloomberg.com, March 11, 2015. Available online: http://www.bloomberg.com/news/articles/2015-03-11/apple-researchkit-sees-thousands-sign-up-amid-bias-criticism
- Wikipedia. Entry Dr Ernest Codman. Available online: https://en.wikipedia.org/wiki/Ernest_Amory_Codman
- Comprehensive care for joint replacement model. Available online: https://innovation.cms.gov/initiatives/cjr
- HCAHPS: patients' perspectives of care survey. Available online: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-instruments/HospitalQualityInits/HospitalHCAHPS.html
- Clinical trials still don’t reflect the diversity of America. NPR News, December 17, 2015. Available online: http://www.npr.org/sections/health-shots/2015/12/16/459666750/clinical-trials-still-dont-reflect-the-diversity-of-america
- Morris ZS, Wooding S, Grant J. The answer is 17 years, what is the question: understanding time lags in translational research. J R Soc Med 2011;104:510-20. [PubMed]
- Wikipedia. Net promoter score. Available online: https://en.wikipedia.org/wiki/Net_Promoter
- The end of theory: the data deluge makes the scientific method obsolete. Wired, June 23, 2008. Available online: http://www.wired.com/2008/06/pb-theory/
- IBM’s Watson now a 2nd year medical student. May 25, 2011. Available online: http://www.forbes.com/sites/bruceupbin/2011/05/25/ibms-watson-now-a-second-year-med-student/
- Steadman I. IBM's Watson is better at diagnosing cancer than human doctors. Wired, Feb 11, 2013. Available online: http://www.wired.co.uk/news/archive/2013-02/11/ibm-watson-medical-doctor
Cite this article as: Weiler A. mHealth and big data will bring meaning and value to patient-reported outcomes. mHealth 2016;2:2.