Enabling breakthroughs in Parkinson’s disease with wearable technologies and big data analytics
Introduction
Parkinson’s disease (PD) is a progressive, degenerative disorder of the central nervous system. PD is the second most common neurodegenerative disease after Alzheimer’s, and is characterized by motor and non-motor symptoms, including tremor, rigidity, bradykinesia, postural instability, depression, dementia, cognitive slowness, and sleep difficulties (1-3).
Currently, the accepted clinical measurement of PD symptom severity is the Unified Parkinson’s Disease Rating Scale (UPDRS) (1,4), which is not optimal for a number of reasons. First, UPDRS assessments are based on subjective reports by patients and semi-objective observations by clinicians. This lack of objectivity allows for variation and potential biases in diagnoses or determinations of PD-related motor states. Second, UPDRS assessments only capture the short, discrete time periods that a patient spends in the clinic with their physician. These snapshots do not necessarily reflect the more variable, day-to-day state of the patient’s PD symptoms in an environment outside of the clinic.
Alternative tools for continuous and objective monitoring of PD motor symptoms are needed to complement clinical assessments and patient reported outcomes. Wearable devices have the ability to collect sensory data, and analysis of this data has the potential to transform our understanding of PD. These devices could offer an objective measure for PD diagnosis and symptoms assessment, provide patients with a tool to monitor and track their disease, and give physicians a more thorough understanding of their patients’ experience with PD, outside the clinic. Attempts to collect and analyze such data (5) have remained limited due to many factors, including difficulties in recruiting and retaining study cohorts, developing complex and robust platforms to store, extract and process ‘big data’ sets, and analyzing and transforming this data into clinically relevant objective measures.
The Michael J. Fox Foundation for Parkinson’s Disease Research and Intel Corporation have joined forces to develop a mobile application and an Internet of Things (IoT) platform to support a large-scale study of objective, continuously sampled sensory data from people with PD (1,6). Currently, our efforts are focused on designing new interactive features to encourage long-term user retention, data capture systems to house massive datasets in a secure fashion, and design of algorithms to provide validated metrics of PD-related motor states, in a way that is useful to researchers, clinicians, and patients.
The IoT platform primarily derives data from users with a consumer, off-the-shelf smartwatch equipped with a 3-axial accelerometer. Users wear this watch and download an app to their personal smartphone, where they can input information about their medication schedule and symptoms. Data streamed from the watch’s accelerometer is transmitted to the cloud for analysis and storage. A data interaction layer allows secure distribution of the data back to users and potentially their clinicians, and an aggregate, de-identified data set will soon be available for distribution to researchers.
Paper objectives
- Provide a high-level blueprint for a scalable IoT platform to collect continuously sampled sensory data.
- Explore the potential value of continuous collection, curation, and dissemination of objective sensory data from people with PD.
- Identify key practical challenges in developing a scalable IoT platform to collect continuously sampled sensory data from people with PD.
An internet of things platform for Parkinson’s disease (PD)
A robust, flexible, and generic IoT platform was built to support the collection of sensory data from wearable devices across multiple protocols and operating systems. Data is passively collected on this platform from users with commercially available wearable devices equipped with sensors that transmit raw data to a smartphone mobile application through Bluetooth. Specifically, users with PD wear a Pebble watch (7) equipped with a 3-axial accelerometer paired to the users’ personal Android-based smartphone (iOS version is currently in development and additional smartwatch options are being integrated into the IoT platform).
The smartphone application also provides users with the opportunity for more active participation in the data collection process. Participants are encouraged to input event markers associated with medication intake, symptom manifestations, and the effect of medication on a variety of structured tasks, which are all linked with the users’ accelerometer data. Through the application’s tailored interface, users can set their medication schedule and receive reminders when it is time to take a medication. Users can also observe objective measures of their own symptoms and activity, directly in the app (see section ‘Data analytics’).
The mobile application runs several analyses on the collected data and transmits the results to a cloud platform. This cloud platform includes a messaging framework (Mosquitto MQTT broker and the AKKA framework that allows distributed parallel processing), big-data storage based on Hadoop, and an application interface layer based on the Play framework. The stored data is distributed back to users, their clinicians, and researchers through a data interaction layer which is built on Apache Phoenix and Spark.
Data analytics
There are two distinctive modes of data analytics within the IoT platform: population analyses and per-patient analyses. Population analyses are applied to users’ data, aggregated as a single data set. These analyses are in development, and will be reported in future publications. The purpose of the per-patient analyses is to transform the raw data to aggregate measures that describe PD motor symptoms of specific patients in a meaningful, interpretable way. Most of the per-patient analyses are run on the user’s smartphones and in the AKKA messaging framework. The following sub-sections describe a selection of the per-patient analyses conducted on the platform.
Gait detection
Existing models for walking detection available in commercial activity trackers (8) are based on data collected from healthy subjects (9). They are not specialized to detect Parkinsonian gait, which may include tremor, dyskinesia, asymmetry, festination, and freezing (10,11). For a PD-specific solution, a dedicated walking detection algorithm was developed based on supervised learning of labeled smartwatch accelerometer data collected from people experiencing a wide variety of PD-related gait disturbances. The raw sensor data is transformed into aggregative features in the time and frequency domains, and a decision tree model is used to classify 5-second intervals as either walking/non-walking.
The model accuracy on a random validation set was 98.5% (Precision 98.9%, Recall 96%), despite the nuances of Parkinsonian gait. The output of the walking detection algorithm is used for calculating a personalized threshold for high activity level (see sub-section ‘Tremor detection’), and as an input to the nighttime activity-tracking algorithm (see sub-section ‘Structured tasks’).
Activity level
The activity level measure is the average value of the absolute acceleration, and was developed to represent the intensity of a user’s activity at any given time. There is a positive correlation between the absolute acceleration and intensity of the activity that is being measured. Since Parkinsonian tremor occurs at a frequency range of 3.5–12 Hz and might result in inaccurate representations of activity level, a low-pass filter with a 3.5 Hz cutoff is applied on the absolute acceleration before averaging it, in order to filter out the potential effect of tremor. The averages are binned into 5 and 30 s intervals.
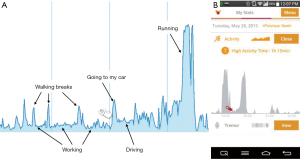
Internal experiments indicate that the activity level algorithm distinguishes not only between ambulatory and non-ambulatory activities, but also the intensity of extensive and non-extensive activity (e.g., running vs. walking; higher-limbs movements vs. static activities, see Figure 1A). A minute-by-minute representation of activity level is displayed on the application (see Figure 1B) and can be correlated with user medication intake events, providing insights about the effect of medication on activity level.
A measure of total high activity time is captured on the app for users each day. This aggregative, personalized measure is defined as the total time of any occurrence of activity that exceeds a threshold that is calculated by averaging a user’s observed activity level during their detected gait sessions. The gait sessions are captured by the tailored gait detection algorithm described in sub-section ‘Activity level’. The daily high activity score is used to benchmark activity levels and serve as a motivator for users to increase their activity goals.
Tremor detection
Parkinsonian tremor is a non-voluntary rhythmic activity characterized by a specific range of frequencies (typically 3.5–12 Hz) that are generally not observed during voluntary activities. Spectral analysis is used to extract activities in this range and occurrences of tremor-like episodes are displayed in daily and monthly graphs on the app. Episodes are correlated to medication intake events entered by the user. Tremor detection is, however, limited to tremors experienced on the hand wearing the watch and insensitive to high frequency activities such as nervous limb movements.
Nighttime activity tracking
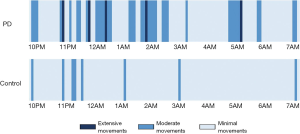
People with PD often suffer from sleep disorders, including insomnia, periodic limb movement disorder and REM-sleep disorder (7,12), which can cause excessive nighttime movement. The nighttime activity feature tracks the intensity and duration of users’ nighttime movements and displays them on the app. Extensive movements may be the result of getting up and walking to the restroom or strong, thrashing movements associated with REM-sleep disorder. Moderate movements may indicate tossing and turning in bed or periodic limb movements, and minimal movements indicate complete rest, either when asleep or awake. Movement intensity is calculated based on the activity level measure and gait detection algorithm, and presented on the app in the form of a nightly graph (Figure 2).
Structured tasks
Structured motor assessments were developed to collect movement data for clinical assessment in a controlled context. For example, a walking assessment evaluates characteristics of a user’s gait, including step count, distance, step time, and body movement symmetry. To conduct the walking assessment, users attach their smartphone to their lower back and walk in a straight line for 10 s. This assessment can be used to measure the effect of medication on gait quality and the progression of symptoms that affect gait over time. Assessment data is stored in the cloud and presented to users on their smartphone app. Data collected from the structured tasks provides researchers with a precise snapshot of users’ movements in specific, predefined contexts. This data is essential to refine current algorithms and inform future algorithm development. In addition, these measures could also be used as novel endpoints in PD clinical trials to measure the effectiveness of a treatment.
Features in the pipeline
The application is already a powerful tool, but its potential is boundless. The app is constantly updated to improve stability, usability and features. In an upcoming release the app will capture users’ instantaneous feedback about medication intake effectiveness through a simple and user-friendly pop-up feature. New algorithms are being developed to measure the occurrence and severity of additional Parkinson’s-related symptoms, including bradykinesia and dyskinesia. We are also collaborating with leading movement disorder specialists to determine how data collected from patients’ experience outside the clinic could transform the standard treatment paradigm. We expect these efforts to increase the utility of the IoT platform and collected data.
Data collection and dissemination
The IoT platform collects and stores data in two different tracks: in-clinic and virtual observational studies. In both tracks, studies received IRB approval and subjects consented to study participation prior to receiving a smartwatch and downloading the mobile application. Data collected from the in-clinic studies were used to develop algorithms for calculating PD measures and to correlate smartwatch sensor data with clinician-measured PD symptom severity. Data collected from the observational studies will be used to analyze daily patterns of medication intake, activity, and symptoms. These unique data sets include raw and aggregated measures of accelerometer data collected during structured activities (e.g., walking in a straight line, folding towels and drinking a glass of water) in and outside the clinic. There is also more than 700,000 h of continuously collected smartwatch data from hundreds of people with PD. These rich data sets continue to grow, and will be shared widely with the research community to develop new algorithms and to categorize the disease into clinically meaningful subtypes.
Engineering and implementation considerations
It’s challenging—but not impossible—to develop a novel healthcare solution that incorporates expertise from multiple sectors and stakeholder groups. The public-private partnership between The Michael J. Fox Foundation, Intel Corporation, clinicians and patient advocates has fostered the development of a technical solution with patient centricity at its core.
Choosing a wearable: medical vs. consumer device
One of the first programmatic decisions made for this project was whether to use a medical- or consumer-grade smartwatch. A key requirement for our device choice is access to raw data measures for novel PD-specific algorithm development. Medical devices provide multiple and more accurate movement (e.g., accelerometer, gyroscope) and vital (e.g., heart rate, skin temperature) measures, but tend to be more expensive. Consumer devices may collect data with less precision, but are more likely to provide an easy and attractive user experience, which in our case was essential to ensure long-term use. Weighing all factors, we implemented the program with a lower-cost consumer device with raw data access. Overall, the device performs sufficiently, but we did encounter complications in smartphone and smartwatch device pairing and battery drainage. We are in the process of testing the capability of alternative consumer- and medical-grade devices with the app.
Soliciting user feedback to foster long-term engagement
For a mobile health solution to scale, the platform must provide value to its data generators (i.e., patients) and data consumers (i.e., researchers, clinicians). Our initial development efforts focused on building a stable data collection and analysis platform to accelerate data sharing. To begin app development, we recruited a group of people with PD to serve as beta-testers. This group provided a much needed patient perspective regarding the app’s features and overall user experience (ease of use, simple flows, clear terminology, etc.). We did not initially implement a long-term patient retention strategy, however this group highlighted the importance of patient engagement in ensuring the collection of high-quality data. We are now incorporating app features that provide value to the user, such as the medication management tool and an easily tuned metronome to help improve the quality of gait. Beta-tester feedback also highlighted the app’s impact on their phone’s battery and data plan usage, which prompted developers to focus efforts toward solving these issues. Overall, beta-tester feedback was instrumental in improving user engagement and subsequent data quality.
Using big data and mobile tech to transform research and patient care
Technology is revolutionizing the way we think about and interact with healthcare. Data scientists can develop algorithms that measure PD symptom severity, and we can validate these measures in a controlled environment. To get the most out of objective measures, however, we need to use them continuously to monitor movement behavior in an environment outside the clinic. Yet, it’s unclear whether patient’s behaviors are the same—or vastly different—in an uncontrolled environment. Our data collection methodology simulates activities of daily living to provide insights into whether these are subtle or vast differences. However, the inherent problem here is how do we validate algorithms measuring at-home behavior when we can’t monitor those behaviors?
For PD and other diseases, it’s still too soon to predict the potential of device-derived objective measures to reveal new insights about the disease, how to ensure they are clinically meaningful, and what the downstream effect will be on research and patient care. As a first step, this project has successfully demonstrated proof of concept that wearable technologies, IoT platforms, and big data offer new and exciting possibilities for more robust, reliable, and low-cost research methodologies and patient care strategies. The extensive database of patient reported outcomes and objective measures collected through IoT platforms are black boxes waiting to be cracked open. We are looking forward to new insights these data will reveal, and are confident that this high-level blueprint has the potential to be scaled beyond PD and into other disease areas, impacting the field and transforming research and patient care.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Gazit E, Bernad-Elazari H, Moore ST, et al. Assessment of Parkinsonian motor symptoms using a continuously worn smartwatch: Preliminary experience. Movement Disorders 2015;30 Suppl 1:688. [PubMed]
- Hughes AJ, Daniel SE, Kilford L, et al. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 1992;55:181-4. [Crossref] [PubMed]
- Jankovic J. Parkinson's disease: clinical features and diagnosis. J Neurol Neurosurg Psychiatry 2008;79:368-76. [Crossref] [PubMed]
- Goetz CG, Tilley BC, Shaftman SR, et al. Movement Disorder Society-sponsored revision of the Unified Parkinson's Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord 2008;23:2129-70. [Crossref] [PubMed]
- Patel S, Lorincz K, Hughes R, et al. Monitoring motor fluctuations in patients with Parkinson's disease using wearable sensors. IEEE Trans Inf Technol Biomed 2009;13:864-73. [Crossref] [PubMed]
- Intel Corporation, Michael J. Fox Foundation. Using wearable technology to advanced Parkinson’s Research. Available online: www.intel.com/content/dam/www/public/us/en/documents/white-papers/using-wearable-technology-mjff.pdf
- Salarian A, Russmann H, Vingerhoets FJ, et al. Gait assessment in Parkinson's disease: toward an ambulatory system for long-term monitoring. IEEE Trans Biomed Eng 2004;51:1434-43. [Crossref] [PubMed]
- Ryu U, Ahn K, Kim E, et al. Adaptive step detection algorithm for wireless smart step counter. Available online: https://www.computer.org/csdl/proceedings/icisa/2013/0602/00/06579332.pdf
- Available online: www.pebble.com
- Moore ST, MacDougall HG, Ondo WG. Ambulatory monitoring of freezing of gait in Parkinson's disease. J Neurosci Methods 2008;167:340-8. [Crossref] [PubMed]
- Sofuwa O, Nieuwboer A, Desloovere K, et al. Quantitative gait analysis in Parkinson's disease: comparison with a healthy control group. Arch Phys Med Rehabil 2005;86:1007-13. [Crossref] [PubMed]
- Partinen M. Sleep disorder related to Parkinson's disease. J Neurol 1997;244:S3-6. [Crossref] [PubMed]
Cite this article as: Cohen S, Bataille LR, Martig AK. Enabling breakthroughs in Parkinson’s disease with wearable technologies and big data analytics. mHealth 2016;2:20.