Non-invasive blood glucose monitoring technology in diabetes management: review
Introduction
Globally, diabetes is a leading non-communicable chronic disease affecting an individual’s health and quality of life (1,2). The International Diabetes Federation reported 463 million adults aged 20–79 years (9.3% of the adult population) had diabetes in 2019 (3,4). Around 79% of adults with diabetes live in developing countries (3), incurring a long-term burden of disease and financial costs (1). Globally, diabetes caused 4.2 million deaths in 2019 (3).
In 2019, over 1.1 million children and adolescents had type 1 diabetes mellitus (T1DM) (3). T1DM is the autoimmune destruction of pancreatic β-cells resulting in insulin deficiency (5). On the other hand, type 2 diabetes mellitus (T2DM) is a chronic metabolic disorder characterised by insulin insensitivity because of insulin resistance, declining insulin production, and eventual pancreatic β-cell failure (6). Ageing, urbanisation, and lifestyle factors have led to an increasing prevalence of T2DM (4). Around 374 million people are at risk of developing T2DM (3).
Inadequate diabetes management increases the risk of comorbidities such as neuropathy (7), nephropathy (8), retinopathy (9), foot disease (10,11), skin disease (12), hearing impairment (13,14), and stroke (15). To maintain health and wellbeing, people with diabetes need to monitor and manage their blood glucose (BG) levels regularly (16).
The management of diabetes involves regular blood tests, which are expensive and inconvenient (6,17). The self-monitoring BG (SMBG) using finger-stick blood samples, test strips, and portable meters has aided diabetes management, especially for those undergoing insulin treatment (18,19). SMBG has several clinical and psychological challenges, such as inflicting finger injury and sensory loss (20), anxiety and fear (21,22), and compromised accuracy and specificity in the readings (19). The discomfort and challenges associated with the SMBG call for novel technology development (23).
Continuous glucose monitoring (CGM) systems are minimally invasive devices that automatically and constantly measure the glucose concentration in the interstitial fluid. Apart from assisting diabetics in managing their BG, CGM assists in the early detection of abnormal glucose regulation, lifestyle optimisation, and optimisation of athletic performance (24). However, CGM systems have limitations, such as inevitable time delay in the transportation of glucose from the blood to the subcutaneous interstitium (approximately 15–20 min), short biosensor lifetime, need for calibration by fingerstick glucose, and limited glucose level readings (25). These challenges direct research toward less-invasive or noninvasive systems (24).
With advancements in technology, non-invasive BG monitoring devices are being developed (26,27). Technologies such as reverse iontophoresis, spectroscopy, ultrasound, electromagnetic sensing, metabolic heat conformation, and other emerging technologies are used for non-invasive BG monitoring (28). Other novel systems, such as biosensor based on polynorepinephrine integrated with a smartphone to function as point-of-care glucose analyser has been proposed (29). Despite considerable research, the progress is slow, and there are currently very minimal devices approved by regulatory organisations worldwide (17).
Recent literature reviewed specific non-invasive technologies such as impedance spectroscopy (30), sweat-based wearable electrochemical sensors (31) and CGM (32), salivary diagnostics (33), and the role of optical, electrical, and breath acetone glucose sensing techniques (34). A study highlighted the clinical significance of non-invasive methods in glucose monitoring and presented the progression of the methods over the years for potential diabetes management (35). Another recent review evaluated the scope of non-invasive technologies in increasing adherence towards checking BG and it concluded that further research is needed to improve the specificity and sensitivity of non-invasive technologies (36).
We systematically reviewed the non-invasive technologies validated against individuals’ BG levels to update the current knowledge of reliable biomarkers, devices, data analysis methods, and factors to be considered in decision-making. Finally, we provide future research direction to design, develop, and deploy SMBG with integrated emerging technologies.
Methods
This section discusses the method followed to retrieve and select the articles considered for this review.
Data sources and search strategy
To undertake our literature search, we used databases, including Embase, MEDLINE, Proquest, Scopus, and Web of Science. The search terms used were (non-invasive OR “non invasive”), AND (diabetics OR diabetes OR “blood sugar” OR “blood glucose”). We applied the search strategy in Table S1 to MEDLINE and customised it for other databases.
Study selection criteria
Table 1 details the applied selection criteria for selecting the articles for this study. We selected English language articles published in peer-reviewed journals between January 2000 and June 2020 (37). We limited the article search timeframe from January 2000, since considerable development, evaluation, approval, and commercialisation of non-invasive diabetes monitoring technologies have been undertaken since then (26). Also, we limited the article search timeframe to June 2020 due to coronavirus disease 2019 (COVID-19). During this period, with recommendations regarding social isolation to minimise the spread of COVID-19, telehealth was the most utilised healthcare delivery method (38). Hence, we have considered the abovementioned period to consider studies using non-invasive technologies that were validated against individuals’ BG levels.
Table 1
| Inclusion criteria |
| (I) Non-invasive glucose monitoring approaches/technologies |
| (II) Non-invasive recordings validated against individual’s blood glucose levels |
| (III) Human subjects (non-invasive technology evaluated on ≥20 diabetes participants) |
| (IV) Participants aged >18 years |
| (V) Peer-reviewed |
| (VI) English language |
| (VII) Year of publication: January 2000–June 2020 |
| Exclusion criteria |
| (I) Publications on incomplete or part of research (e.g., editorials, abstracts, workshop/conference summaries, research proposals, descriptive surveys, clinical protocols, research methods, literature reviews, conceptual papers) |
| (II) Non-invasive glucose monitoring approaches/technologies evaluated on only non-diabetes participants |
| (III) Non-human focused (e.g., animals) |
| (IV) Diabetes treatment, gestational diabetes |
| (V) Non-invasive monitoring of other disease severity due to diabetes |
| (VI) Evaluation and development of research tools (e.g., hardware and algorithm improvement studies, clinical measurement technology to access and analyse secondary data, prototypes, and simulations) |
| (VII) Data modelling & dataset analysis—application of machine learning algorithms to predict diabetes, data modelling and statistical analysis of diabetes detection, diabetes risk prediction |
During the early stage of non-invasive technology development, there is a need to assess the specificity, sensitivity, and user satisfaction among diabetes individuals and healthcare providers. Hence, we have strictly considered studies that evaluated non-invasive technologies against an individual’s BG levels, including haemoglobin A1c (HbA1c), fasting plasma glucose (FPG), and 2-hour PG during an oral glucose tolerance test (OGTT) (36,39).
Studies with a small sample size could negatively influence the outcomes (40), hence we have considered studies with at least 20 diabetes participants. Since the studies used non-invasive technologies that are under development and would need informed consent, we considered studies with adult participants (41).
Study selection process
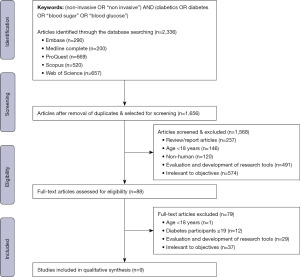
Figure 1 illustrates the article selection process. The search keywords were entered into the database, and we imported the selected article citations into the reference management software EndNote. The duplicate articles were eliminated and a total of 1,656 articles were considered for screening.
The first level screening was performed by reading the title and abstract of the article. We excluded reviews and editorials, studies undertaken on those younger than 18 years, non-human subjects, and research tool evaluation and development. Further, we excluded studies irrelevant to the objective of this review, such as research proposals, descriptive surveys, clinical protocols, research methods, conceptual papers, undertaken among non-diabetic participants, hardware and algorithm improvement studies, clinical measurement technology to access and analyse secondary data, prototypes, simulations, application of machine learning algorithms to predict diabetes, data modelling and statistical analysis of diabetes detection, and diabetes risk prediction. We obtained 88 articles for further full-text review at the end of this stage.
At the second level of screening, we read the entire article and eliminated studies undertaken in non-adults, less than 20 participants, and those irrelevant to the objective of this review. We then had nine articles that we considered for this review.
Data extraction
A reviewer (J.C.M.) independently evaluated the titles and abstracts of all articles identified in the initial database search and were discussed with other authors (S.M.S.I. and S.A.); then, J.C.M. reviewed the full text for eligibility according to the study’s inclusion criteria (42). The reviewer then extracted study characteristics, including country of study, participant count, study duration, and patient characteristics, including diabetes type, age, and gender. Also, we obtained the details regarding non-invasive devices, technology, parameters recorded, and the duration of observation.
Analysis of selected studies
We analysed the selected studies through the theoretical lens of the Technology Task Fit theory (43). This theory conceptualises the fit between a technology (in this case, non-invasive technologies) and the task it aims to support (i.e., reading the glucose level in human blood) through task requirements, technical characteristics and performance impacts (clinical standards) and utilisation (BG monitoring and management).
In this review, technology characteristics include non-invasive devices, technology, parameters recorded, and the duration of observation. Task requirements include technically robust, clinically accurate, with clinical and safe interfaces, easy to use, alleviating psychological effects, and economically affordable (16). To critically analyse each study using technology-task fit performance, we considered their statistically significant value with a cut-off point of the results as designated by P values at the level of α<0.05.
Results
The initial search returned 2,336 articles. After removing duplicates, we included 1,656 articles for the title and abstract screening. Further, we applied selection criteria to the obtained 88 articles and finally selected nine studies in this review.
The studies were undertaken in China (44), India (45-47), Israel (48), Japan (49), the Philippines (50,51), and the United States of America (52). Table 2 details the study and participant characteristics.
Table 2
| Study | Country | Count | T1DM and T2DM | Non-DM | Age (years) | Male (%) | Female (%) | Validated against |
|---|---|---|---|---|---|---|---|---|
| Saliva | ||||||||
| Tiongco, 2019 (51) | Philippines | 80 | Total: 25 | 55 | 18–NS | NS | NS | FBG |
| Malik, 2019 (47) | India | 175 | T2DM: 88 | 87 | 18–69 | 50 | 50 | FBG |
| Tiongco, 2018 (50) | Philippines | 75 | T2DM: 75 | – | 31–61 | 33 | 67 | FBG |
| Sai Archana, 2016 (45) | India | 75 | T2DM: 50 | 25 | 40–70 | NS | NS | FBG, HbA1c |
| Skin | ||||||||
| Lin, 2018 (48) | Israel | 114 | T2DM: 114 | – | 18–NS | NS | NS | HbA1c |
| Sivanandam, 2013 (46) | India | 60 | Total: 29 | 31 | 19–75 | 45 | 55 | HbA1c |
| Sheng, 2011 (44) | China | 195 | Total: 75 | 120 | DM: 60.5±8.4; Non-DM: 47.0±16.2 | 51 | 49 | HbA1c |
| Tierney, 2001 (52) | USA | C: 231 | T1DM: 151 | 80 | 48.2±15.0 | NS | NS | Fingerstick |
| H: 124 | T1DM: 74 | 50 | 46.5±11.7 | |||||
| Breath | ||||||||
| Takemoto, 2019 (49) | Japan | 36 | Total: 20 | 16 | 20–82 | 58 | 42 | HbA1c |
Age: data are presented as range or mean ± SD. T1DM, type-1 diabetes mellitus; T2DM, type-2 diabetes mellitus; non-DM, non-diabetes mellitus; NS, not specified; FBG, fasting blood glucose; HbA1c, haemoglobin A1c; C, clinical environment; H, home environment; SD, standard deviation.
The number of study participants varied between 36 (49) and over 300 (52). The study settings differed, i.e., the clinical studies were performed after an overnight fast (44,45,47,49-51), for an extensive duration in a day (46,52), and a few days (48). Also, a study evaluated non-invasive technology in home settings (52).
The participant selection criteria varied between the studies. For example, studies excluded participants living with chronic diseases, including T1DM (52) and T2DM (45,47,48,50). A few studies did not mention the participant’s diabetes type (44,46,49,51). Studies included non-diabetes participants (44-47,49,51,52). The HbA1c (44-46,48,49,52), or fasting BG (FBG) (45,47,50,51) recordings categorised participants as diabetes and non-diabetes.
The participant’s age varied between 18 (47,48,51) and 82 years (49). The studies that evaluated saliva (45,47,50,51) to screen, diagnose, and monitor diabetes had participants aged between 18 (51) and around 70 years (45,47). Whereas studies examining technologies on the skin (44,46,48,52) to estimate BG levels had participants aged between 18 (48) and 75 years (46). Furthermore, a wide age range (20–82 years) is observed in a study assessing breath samples to screen for diabetes (49). Nevertheless, a few studies failed to explicitly mention the oldest participant’s age (48,51).
Studies have reported the participant’s age in each group (i.e., diabetes and non-diabetes) and their relationship with HbA1c (46), and the accuracy of device recordings (44). A study estimated BG by non-invasive infra-red (IR) thermography, which is the process of using a thermal imager to detect heat emitted from an object, converting it to temperature and displaying an image of the temperature distribution observed a significant (P=0.002) age difference between diabetes (n=29, age: 52±15 years) and non-diabetes subjects (n=31, age: 40±11 years) (46). Additionally, a positive correlation (r=0.504, P<0.01) between age and HbA1c is observed (46).
A study on iontophoresis technology (EZSCAN), which is a technique that removes molecules from within the body for detection, observed a significant (P=0.0001) age difference between diabetic (n=75, age: 60.5±8.4 years) and non-diabetic (n=120, age: 47.0±16.2 years) participants (44). Also, the study observed a statistically significant (P≤0.003) relationship in the sensitivity and specificity between older (age: ≥54 years, n=95, sensitivity: 93%, specificity: 24%) and younger (age: <54 years, n=100, sensitivity: 61%, specificity: 83%) subjects at diabetes index ≥40 as EZSCAN threshold (44). Age is a significant parameter in estimating the BG value from saliva (47) and thermography (46) but not with other measured parameters (49,50,52).
There was an equal representation of male and female participants in some studies (44,46,47). However, a few studies had skewed male (49) and female (50) participants. A few studies have not specified the gender percentage (45,48,51,52). Furthermore, studies observed that gender is insignificant in estimating FBG value from saliva (47). Likewise, another study evaluating the iontophoresis technology witnessed similar sensitivity and specificity in both sexes (P≥0.500) (44).
Non-invasive screening
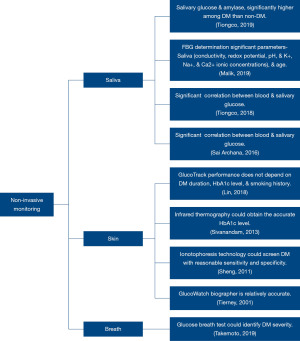
Table 3 represents the details of non-invasive technologies considered in this review. Figure 2 depicts the non-invasive technologies and significant outcomes of the assessed studies.
Table 3
| Article | Device | Technology | Parameters recorded | Procedure duration |
|---|---|---|---|---|
| Saliva | ||||
| Tiongco, 2019 (51) | Not specified | Laboratory procedure | Glucose, amylase, calcium, & phosphorus | After overnight fasting |
| Malik, 2019 (47) | • F-71 pH/ORP meter | Not specified | Conductivity, redox potential, pH, calcium, potassium & sodium ionic concentrations | |
| • Horiba Laqua | ||||
| Tiongco, 2018 (50) | Not specified | Not specified | Glucose | |
| Sai Archana, 2016 (45) | • Freestyle precision | Not specified | Glucose, pH, potassium & sodium ionic concentrations | |
| • D-10 hemoglobin testing system | ||||
| • Roche 9180 electrolyte analyzer | ||||
| • Optima 1 | ||||
| Skin | ||||
| Lin, 2018 (48) | GlucoTrack | Ultrasonic, electromagnetic & thermal | Glucose fluctuation in the earlobe tissue | • Day 1: device calibration (20–30 min) |
| • 2–3 non-consecutive days: samples (16 readings/day), 8–10 hours | ||||
| • 1 minute/test | ||||
| Sivanandam, 2013 (46) | FLIR T 400 | Infrared thermography | Skin temperature at eye, ear, forehead, neck, upper and lower extremities | • 15 min—acclimatisation time |
| • Image capture time | ||||
| Sheng, 2011 (44) | EZSCAN | Reverse iontophoresis | Electrochemical Conductance at forehead, hands, and feet |
2 min test |
| Tierney, 2001 (52) | GlucoWatch | Reverse iontophoresis | Glucose | Every 20 min for 12 hours |
| Breath | ||||
| Takemoto, 2019 (49) | Not specified | Non-dispersive infrared isotope spectrometry | Three types of glucose breath tests | At baseline and 10-min intervals over 150 min |
Saliva
Saliva is a biofluid that could be retrieved effortlessly, having a proportional relationship to the BG level; hence is evaluated for diabetes diagnostic applications (47). All studies collected blood and saliva samples after an overnight fast (45,47,50,51). However, the methodology and analysis of saliva varied between the studies. For example, unstimulated saliva was collected and analysed for salivary glucose and minerals (51), electrochemical properties and minerals (47), and salivary flow rate, levels of glucose, and minerals (45). A study collected saliva after rinsing the mouth with distilled water two times before glucose was estimated (50). The participants were T2DM (45,47,50) and a study did not specify the diabetes type (51).
A study clinically evaluated the potential utility of salivary glucose, amylase, calcium, and phosphorus as a non-invasive diagnostic marker and observed that only salivary glucose (r=0.416, P<0.001) and salivary amylase (r=0.226, P=0.040) had a positive correlation with FBG having good potential in discriminating diabetics from non-diabetics (51). Likewise, another study observed a statistically significant (P=0.005) relationship between FBG and salivary glucose levels (45). Furthermore, among the considered participants, a statistical (P=0.01) correlation between saliva and FBG is observed in the uncontrolled diabetic patient group (HbA1C level >7.0%) (45).
The electrochemical properties of saliva such as conductivity, redox potential, pH and K+, Na+ and Ca2+ ionic concentrations, and participants’ age and gender were trained and tested using a mathematical regression algorithm to estimate FBG (47). On analysis, NeuralNet Boosting Regression (NBR: 87.4%±1.7%) presented the best-classifying accuracy when compared with Kernel Ridge Regression (KRR: 73.8%±1.9%) and Neural Network Regression (NNR: 84.7%±2.1%) (47). Whereas NNR (93.3%±1.7%) had better sensitivity when compared with NBR (88.7%±2.1%) and KRR (83.1%±0.4%) (47). Furthermore, the study developed an integrated system using sensors and Arduino UNO R3, and on evaluating the accuracy considering different samples, NBR yielded an accuracy of 81.67%±2.53% (47). Additionally, on validating the performance of the developed interface, 80% of the data lie in the A zone while 20% are in the B zone according to Clark Error Grid (CEG) analysis (47).
A study using saliva sample observed a statistically significant (r=0.715, P=0.001) relationship between saliva and FBG among T2DM participants (50). Furthermore, by applying linear regression analysis, salivary glucose (P<0.001) could predict the value of BG (50).
Skin
Skin is a human body barrier with physicochemical characteristics differing among individuals due to factors such as age, sex, race, anatomical area of the skin, the intensity of perspiration, skin temperature and ambient temperature, air humidity, season of the year, daily rhythm, hormonal balance, and many others (53). To estimate the BG levels, different non-invasive technologies such as fluorescence, optical polarimetry, optical coherence tomography, different types of spectroscopy, metabolic heat conformation, millimetre and microwave sensing, electromagnetic sensing, ultrasound, sonophoresis, and reverse iontophoresis are being evaluated (26).
The non-invasive devices used technologies such as reverse iontophoresis (44,52), infrared thermography (46), and a combination of ultrasonic, electromagnetic, and thermal technology (48) to estimate BG levels. The devices were calibrated and worn at the wrist (52) and attached to the earlobe (48). Moreover, the study was undertaken in clinical settings and at-home settings for five days (52). The captured recordings were downloaded and analysed offline, along with other demographic parameters such as age, height, and weight (48,52).
A study using infrared thermography captured images of body parts including the forehead, inner eye canthus, neck region, tympanic region of the ear, carotid region, palm, knee, and the tibial region, at a distance of 1 meter from the subject (46). Whereas, in a study using reverse iontophoresis technology, the participant stood still with the headband on the forehead and their hands and feet on electrode pads for 2 min, during which the electrochemical conductance (EC) to derive diabetes index considering sex, age, body mass index, and systolic blood pressure (SBP) is calculated (44). Although the technologies differed, the studies established a relationship to estimate BG levels non-invasively.
Due to glucose-related shifts in ion concentration, density, compressibility and hydration of both cellular and extracellular compartments of the tissue, there could be glucose variations in the earlobe tissue, which may further vary due to microvascular complications, such as diabetes duration, HbA1c level, and smoking history (48). A study evaluated the earlobe tissue parameters against actual BG levels and found 98.0% of the measurements were in the clinically acceptable zones A and B, with 52.3% in the clinically accurate zone A on performing CEG analysis (48). Additionally, the non-invasive device accuracy was comparable across individuals with different clinical characteristics such as diabetes duration and smoking history, indicating it is suitable for various people with T2DM (48).
The core body temperature of T1DM and T2DM participants vary from non-diabetics and could also be adversely affected due to thermal stress (i.e., hot and cold exposure) (54). On analysing the thermal images of diabetes (n=29) and non-diabetes (n=31), the skin temperature of the body regions indicates a significant decrease in the surface temperature at the inner canthus of the eye (diabetes: 35.56±0.47 ℃, non-diabetes: 36.01±0.43 ℃, P=0.000, 95% CI: 0.22–0.69); knee region (diabetes: 31.68±0.65 ℃, non-diabetes: 32.37±0.94 ℃, P=0.002, 95% CI: 0.27–1.11); tibial region (diabetes: 32.20±0.83 ℃, non-diabetes: 32.82±0.77 ℃, P=0.004, 95% CI: 0.2–1.03) and at the forehead (diabetes: 34.68±0.39 ℃, non-diabetes: 35.00±0.48 ℃, P=0.006, 95% CI: 0.09–0.55) region (46).
A positive correlation between HbA1c and age (r=0.504, P<0.01), SBP (r=0.305, P<0.05), and diastolic blood pressure (DBP) (r=0.278, P<0.05) respectively is observed (46). Whereas, a negative correlation between HbA1c and core body temperature measurement at the inner canthi of the eye (r=−0.462, P<0.01), skin surface temperature at the knee region (r=−0.267, P<0.05) is observed (46). Additionally, an optimal regression model (r=0.643, P=0.000) to estimate the HbA1c (mmol/mol) was developed by considering the age (years); skin surface temperature (℃) coefficients measured at the carotid and the knee region (46).
Reverse iontophoresis technology estimates BG level by performing spot checks (44) and monitoring continuously (52). In the study using reverse iontophoresis technology to measure EC (micro Siemens, µSi), the EC was significantly (P<0.001), lower in diabetes patients at the hands (44 vs. 61 µSi) and feet (51 vs. 69 µSi) locations, but not at the forehead (15 vs. 17 µSi, P=0.39) (44). Furthermore, when the diabetes index threshold was set as 40 (the manufacturer suggested), the sensitivity and specificity for the diagnosis of diabetes were 85% and 64%, respectively (44).
While using reverse iontophoresis technology to monitor continuously, it was observed that the mean difference between biographer and finger-stick measurements was −0.01 and 0.26 mmol·L−1 for the clinical (n=231) and home environments (n=124), respectively (52). Likewise, the mean absolute value of the relative difference was 1.06 and 1.18 mmol·L−1 for the clinical and home environments, respectively (52). Furthermore, over 94% of the biographer readings obtained in both studies were in the clinically acceptable A + B region of the CEG (52).
Breath
Human breath has various components associated with pathological state and could be used potentially as a tool for the diagnosis and study of medical diseases (17). After an overnight fast, to evaluate [1,2,3-13C] glucose present in the breath, the patients received 100 mL of water containing 100 mg of 13C-glucose; following that, breath samples were taken at baseline and at 10-min intervals over 150 min to perform 1-13C, 2-13C, and 3-13C glucose breath test (49). The peak value of 13CO2 (Cmax), is observed to be low in diabetes patients compared to the non-diabetes for the tests 1-13C (diabetes: 7.0±1.9, non-diabetes: 10.4±1.6, P<0.001), 2-13C (diabetes: 7.5±2.5, non-diabetes: 11.3±1.8, P<0.001), and 3-13C (diabetes: 11.3±3.5, non-diabetes: 14.8±3.0, P=0.007) (49).
A significant difference was observed between diabetes and non-diabetes participants for the breath tests, 1-13C (diabetes: 642±210, non-diabetes: 914±226, P<0.001), 2-13C (diabetes: 717±281, non-diabetes: 1174±178, P<0.001), and 3-13C (diabetes: 1,289±409, non-diabetes: 1,668±400, P=0.016) (49). The results suggested that the 1-13C glucose breath test is suitable for patients with late-stage diabetes, whereas the 2-13C glucose breath test is ideal for early-stage diabetes (49).
Discussion
This study reviewed non-invasive technologies validated against individual BG levels among people with diabetes. Studies appraising the accuracy of saliva (45,47) and thermography (46) to estimate BG levels were cost-effective technologies. In contrast, a study evaluating the breath sample found the technology costly and time-consuming to calculate BG levels non-invasively (49). Hence there is an imperative need to develop screening tools that are demonstrably simple, valid, reliable, quick to administer, and easy to use (55).
Ageing increases diabetes risk (56), and globally, 20% of adults above 65 years are diabetic (3). The risk of diabetes is high among men compared with women (57). However, a few studies have considered participants above 65 years (45-47,49), whereas other studies included younger participants (44,50,52) or have not mentioned the age limit (48,51). Studies have considered participants with more women (39) and have not specified gender (37,40,41,44). It is evident that diabetes risk is high among the elderly and men and is highly prevalent in developing countries (4,56,57). Consequently, interpreting the current results of the studies need to be done with caution.
Studies evaluated the reliability of the readings against gold-standard but failed to examine the acceptance and usability of the technology among various stakeholders, including clinicians and participants (44-52). Hence there is an imperative need to develop secure, reliable, socially acceptable, cost-effective, and usable systems by various stakeholders, i.e., the system must support different user (gender, socio-economic) groups to interact effectively and quickly, and also facilitate older people to access the system effortlessly (58).
The symptoms presented by T1DM and T2DM patients and their physiological conditions differ (59,60). However, a few studies have not mentioned diabetes type among the participants (44,46,49,51). Furthermore, diabetes is increasing and often undiagnosed in developing countries and among certain ethnic groups, including South Asians, due to developing metabolic abnormalities at a younger age (61). Hence, non-invasive technologies shall be evaluated among T1DM and T2DM, considering demographic and behavioural factors to achieve a generalisable result. Additionally, there is a need to take decisive actions to prevent and manage diabetes using innovative and low-cost approaches (61).
Promising non-invasive approaches
The advancements in science and technology are facilitating the development of novel non-invasive BG measuring systems. A few optical measurements that are considered are near-infrared and mid-infrared spectroscopy, optical polarimetry, Raman spectroscopy, fluorescence method, and optical coherence tomography. Microwave sensors have a broad development prospect due to their high penetration depth, non-ionisation, low cost, and portability. Electrochemical methods, such as reverse iontophoresis technology, and biofluid, including saliva, tears, and sweat-based sensor systems are being developed to monitor glucose (62).
Commercial BG measuring devices are in the market, however, due to either product discontinuation or failure in receiving the Food and Drug Administration or Conformite Europeenne approval, only a few are available. Two dominant commercial systems, namely, FreeStyle Libre and Dexcom provide continuous glucose measurement and work for at least ten days. Nevertheless, both use minimal-invasive technology resulting in disadvantages, such as infection risk, pain for the patient, and the cost of sensor replacement. Much research is needed to develop less costly, more convenient, and more accurate glucose measurement and monitoring devices (63).
Challenges in non-invasive approaches
Non-invasive glucose monitoring system encounters several challenges that compromise the accuracy of the system. For example, optical sensors struggle in obtaining good measurement precision, low signal-to-noise ratios and the management of motion artefacts (64). Likewise, sweat-based glucose sensing still faces many challenges, such as difficulty in sweat collection, activity variation of glucose owing to lactic acid secretion and changes in ambient temperature, and delamination of the enzyme when exposed to mechanical friction and skin deformation (62).
Safety and regulatory approvals of non-invasive approaches
SMBG using finger-stick blood samples, test strips, and portable meters has aided diabetes management (18,19); but has several safety challenges, such as inflicting finger injury and sensory loss (20), anxiety and fear of self-injecting (21,22), and compromised accuracy and specificity in the readings (19). On the other hand, CGM systems are minimally invasive devices that automatically and constantly measure the glucose concentration in the interstitial fluid (24); but have safety and accuracy concerns due to painful insertion, skin/adhesive problems, the necessity of calibration by fingerstick glucose, the need of avoiding calibration timing after eating, and limited glucose levels readings (25,65).
Non-invasive systems are developed integrating various technologies that could compromise patient safety. For example, iontophoresis has benefits in diabetes management as it is non-invasive and could bypass the first-pass metabolism; however, there are safety concerns about unintended overdose (66). Also, ultrasound could have safety issues on human tissue if it is accompanied by a liquid or solid placed between the device that emits the ultrasound and the body tissue (67). The safety of magnetic fields, including static magnetic fields, extremely low-frequency electromagnetic fields and pulsed electromagnetic fields is still widely discussed and considered (68).
As substantiated by the literature, diabetes risk is high among men, the elderly, and those living in developing countries (4,56,57). Hence, there is a need to formulate guidelines to evaluate non-invasive glucose monitoring technologies to report participants’ diabetes type and duration, accuracy, reliability, accessibility, safety, security, privacy, convenience of use, and cost-effectiveness, to consider it globally. Also, the different technologies used to develop non-invasive systems could cause safety issues. Hence, appropriate regulatory guidelines are to be formed to oversee the development of non-invasive systems.
Implications for research
Several non-invasive technologies are at an initial stage of development (17). There is a statistical relationship between standard clinical BG measurement obtained and non-invasive methods included in this review; thus, offering potential clinical use.
From the Diffusion of Innovation theoretical perspective, we suggest further research to advance the uptake of non-invasive technologies by early adopters through different communication channels to facilitate innovative decision-making (69). From the Technology Task Fit theoretical perspective, we recommend establishing the fidelity (fit) between non-invasive technologies and healthcare practice (43).
While the results promise clinical validity, other factors such as safety and ease of use, psychological effects, and economic affordability should be evaluated. Specifically, the severity of diabetes varies due to the type and duration of diabetes (59,60). Moreover, such a perspective would enable more tailoring of the non-invasive solution for different sub-groups in the community so that the usefulness of the specific solution can then be maximised which in turn will be more likely to lead to frequent use and hopefully better managed diabetes.
In addition, we can integrate non-invasive technologies with digital healthcare to be more accessible and efficient and thus also address a value-based healthcare paradigm and support a healthcare value proposition of better access, quality and value of care delivery.
Future research direction
With the commercialisation of 5G technology and smartphones with higher performance and the capability to integrate with other peripherals, there is a scope to deliver personalised healthcare solutions integrated with alternative technologies such as autonomous vehicles, artificial intelligence (AI), and the internet of things (IoT) (70-72). Since the global market is poised for rapid growth, including in developing nations and could have widespread implications in delivering personalised healthcare (73-75), interoperable mHealth solutions shall be designed and developed (76).
Our future work focuses on exploring and realising the potential of mHealth solutions integrated with data analytics, autonomous vehicles, AI, and IoT to deliver remote personalised diabetes monitoring and management solutions.
The health and wellbeing of diabetic individuals revolve around significant lifestyle changes and meticulous attention and monitoring by the patient and health professionals (16). Furthermore, interventions provided through digital technology have yielded positive outcomes in patients’ diabetes management (1,77-79).
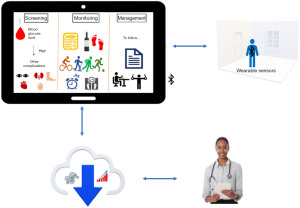
Big data analytics could assist in delivering personalised healthcare (80-82). Figure 3 illustrates the mHealth diabetes management process integrated with data analytics. There are research prospects to design, develop, and deploy integrated diabetes management systems with mobile technologies, data analytics, and IoT to deliver a personalised healthcare system that healthcare professionals could access and assess remotely to manage and monitor patients’ health with ease.
Limitations of this review
This review had a few limitations. First, the applied selection criteria resulted in selecting only nine studies for analysis. Second, this review represents a small proportion of non-invasive technologies used in glucose monitoring due to the selection criteria applied to select studies evaluated clinically with credibility. Third, studies with a sample size of less than 100 (45,46,49-51) were undertaken in developed and developing countries and had varying age and gender participants. Although the findings could be extended to large sample sizes and different populations to validate the results’ accuracy, the current findings might not be generalisable. Finally, the heterogeneity in data has restricted us from conducting a meta-analysis.
Conclusions
Non-invasive glucose monitoring technologies showed a statistical relationship between BG measurements obtained against standard clinical measures. Formulating regulatory guidelines could foresee the deployment of approved non-invasive BG monitoring technologies in healthcare practice. Opportunities exist for future research to advance research progress and facilitate early technology adoption for healthcare practice. Moreover, there are research prospects to design, develop, and deploy integrated diabetes management systems with mobile technologies, data analytics, and the IoT to deliver a personalised monitoring system.
Acknowledgments
Funding: This study was supported by an Emerging Leadership Fellowship from the National Health and Medical Research Council of Australia (No. APP1195406 to S.M.S.I) and Vanguard grants from the National Heart Foundation of Australia (to S.M.S.I).
Footnote
Peer Review File: Available at https://mhealth.amegroups.com/article/view/10.21037/mhealth-23-9/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://mhealth.amegroups.com/article/view/10.21037/mhealth-23-9/coif). S.M.S.I serves as an unpaid editorial board member of mHealth from May 2023 to April 2025. S.M.S.I reports support for the present manuscript from an Emerging Leadership Fellowship from the National Health and Medical Research Council of Australia (APP1195406) and Vanguard grants from the National Heart Foundation of Australia; S.M.S.I reports unpaid leadership roles outside the submitted work with the IT Committee of the Cardiac Society of Australia and New Zealand, ESC Heart Failure Association as a volunteer on the Cardiac Devices Committee and with WHO-ITU Global Initiative on AI for Health. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Islam SMS, Peiffer R, Chow CK, et al. Cost-effectiveness of a mobile-phone text messaging intervention on type 2 diabetes—A randomized-controlled trial. Health Policy Technol 2020;9:79-85. [Crossref]
- Wickramasinghe N, John B, George J, et al. Achieving Value-Based Care in Chronic Disease Management: Intervention Study. JMIR Diabetes 2019;4:e10368. [Crossref] [PubMed]
- Kubben P. Chapter 12 Mobile Apps. In: Kubben P, Dumontier M, Dekker A, editors. Fundamentals of Clinical Data Science. Cham (CH): Springer; 2019.
- Saeedi P, Petersohn I, Salpea P, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Res Clin Pract 2019;157:107843. [Crossref] [PubMed]
- Kahanovitz L, Sluss PM, Russell SJ. Type 1 Diabetes - A Clinical Perspective. Point Care 2017;16:37-40. [Crossref] [PubMed]
- Olokoba AB, Obateru OA, Olokoba LB. Type 2 diabetes mellitus: a review of current trends. Oman Med J 2012;27:269-73. [Crossref] [PubMed]
- Bansal V, Kalita J, Misra UK. Diabetic neuropathy. Postgrad Med J 2006;82:95-100. [Crossref] [PubMed]
- Lim AKh. Diabetic nephropathy - complications and treatment. Int J Nephrol Renovasc Dis 2014;7:361-81. [Crossref] [PubMed]
- Shah AR, Gardner TW. Diabetic retinopathy: research to clinical practice. Clin Diabetes Endocrinol 2017;3:9. [Crossref] [PubMed]
- Perrin BM, Allen P, Gardner MJ, et al. The foot-health of people with diabetes in regional and rural Australia: baseline results from an observational cohort study. J Foot Ankle Res 2019;12:56. [Crossref] [PubMed]
- Al-Rubeaan K, Al Derwish M, Ouizi S, et al. Diabetic foot complications and their risk factors from a large retrospective cohort study. PLoS One 2015;10:e0124446. [Crossref] [PubMed]
- de Macedo GM, Nunes S, Barreto T. Skin disorders in diabetes mellitus: an epidemiology and physiopathology review. Diabetol Metab Syndr 2016;8:63. [Crossref] [PubMed]
- Horikawa C, Kodama S, Tanaka S, et al. Diabetes and risk of hearing impairment in adults: a meta-analysis. J Clin Endocrinol Metab 2013;98:51-8. [Crossref] [PubMed]
- Kim MB, Zhang Y, Chang Y, et al. Diabetes mellitus and the incidence of hearing loss: a cohort study. Int J Epidemiol 2017;46:717-26. [Crossref] [PubMed]
- Chen R, Ovbiagele B, Feng W. Diabetes and Stroke: Epidemiology, Pathophysiology, Pharmaceuticals and Outcomes. Am J Med Sci 2016;351:380-6. [Crossref] [PubMed]
- Nguyen L, Adibi S, Wickramasinghe N. Towards a Better Life for Diabetic Patients: Developing and Integrating a Non-invasive Self-Management Support Tool Within a Smart Digital Companion. In: Wickramasinghe N, Bodendorf F, editors. Delivering Superior Health and Wellness Management with IoT and Analytics. Healthcare Delivery in the Information Age. Cham: Springer; 2020:207-22.
- Minh Tdo C, Blake DR, Galassetti PR. The clinical potential of exhaled breath analysis for diabetes mellitus. Diabetes Res Clin Pract 2012;97:195-205. [Crossref] [PubMed]
- Standards of medical care in diabetes--2011. Diabetes Care 2011;34:S11-61. [Crossref] [PubMed]
- Olansky L, Kennedy L. Finger-stick glucose monitoring: issues of accuracy and specificity. Diabetes Care 2010;33:948-9. [Crossref] [PubMed]
- Le Floch JP, Bauduceau B, Lévy M, et al. Self-monitoring of blood glucose, cutaneous finger injury, and sensory loss in diabetic patients. Diabetes Care 2008;31:e73. [Crossref] [PubMed]
- Shlomowitz A, Feher MD. Anxiety associated with self monitoring of capillary blood glucose. Br J Diabetes 2014; [Crossref]
- Al Hayek AA, Robert AA, Babli S, et al. Fear of Self-Injecting and Self-Testing and the Related Risk Factors in Adolescents with Type 1 Diabetes: A Cross-Sectional Study. Diabetes Ther 2017;8:75-83. [Crossref] [PubMed]
- Heinemann L. Finger pricking and pain: a never ending story. J Diabetes Sci Technol 2008;2:919-21. [Crossref] [PubMed]
- Holzer R, Bloch W, Brinkmann C. Continuous Glucose Monitoring in Healthy Adults-Possible Applications in Health Care, Wellness, and Sports. Sensors (Basel) 2022;22:2030. [Crossref] [PubMed]
- Sun MT, Li IC, Lin WS, et al. Pros and cons of continuous glucose monitoring in the intensive care unit. World J Clin Cases 2021;9:8666-70. [Crossref] [PubMed]
- Villena Gonzales W, Mobashsher AT, Abbosh A. The Progress of Glucose Monitoring-A Review of Invasive to Minimally and Non-Invasive Techniques, Devices and Sensors. Sensors (Basel) 2019;19:800. [Crossref] [PubMed]
- Tura A, Maran A, Pacini G. Non-invasive glucose monitoring: assessment of technologies and devices according to quantitative criteria. Diabetes Res Clin Pract 2007;77:16-40. [Crossref] [PubMed]
- Vashist SK. Non-invasive glucose monitoring technology in diabetes management: a review. Anal Chim Acta 2012;750:16-27. [Crossref] [PubMed]
- Jędrzak A, Kuznowicz M, Rębiś T, et al. Portable glucose biosensor based on polynorepinephrine@magnetite nanomaterial integrated with a smartphone analyzer for point-of-care application. Bioelectrochemistry 2022;145:108071. [Crossref] [PubMed]
- Huang J, Zhang Y, Wu J. Review of non-invasive continuous glucose monitoring based on impedance spectroscopy. Sens Actuators A Phys 2020;311:112103. [Crossref]
- Mohan AMV, Rajendran V, Mishra RK, et al. Recent advances and perspectives in sweat based wearable electrochemical sensors. Trends Analyt Chem 2020;131:116024. [Crossref]
- Zafar H, Channa A, Jeoti V, et al. Comprehensive Review on Wearable Sweat-Glucose Sensors for Continuous Glucose Monitoring. Sensors (Basel) 2022;22:638. [Crossref] [PubMed]
- Pappa E, Vougas K, Zoidakis J, et al. Proteomic advances in salivary diagnostics. Biochim Biophys Acta Proteins Proteom 2020;1868:140494. [Crossref] [PubMed]
- Shokrekhodaei M, Quinones S. Review of Non-invasive Glucose Sensing Techniques: Optical, Electrical and Breath Acetone. Sensors (Basel) 2020;20:1251. [Crossref] [PubMed]
- Laha S, Rajput A, Laha SS, et al. A Concise and Systematic Review on Non-Invasive Glucose Monitoring for Potential Diabetes Management. Biosensors (Basel) 2022;12:965. [Crossref] [PubMed]
- Bolla AS, Priefer R. Blood glucose monitoring- an overview of current and future non-invasive devices. Diabetes Metab Syndr 2020;14:739-51. [Crossref] [PubMed]
- Moses JC, Adibi S, Shariful Islam SM, et al. Application of Smartphone Technologies in Disease Monitoring: A Systematic Review. Healthcare (Basel) 2021;9:889. [Crossref] [PubMed]
- Scott ES, Jenkins AJ, Fulcher GR. Challenges of diabetes management during the COVID-19 pandemic. Med J Aust 2020;213:56-57.e1. [Crossref] [PubMed]
- Brar PC. Update on the current modalities used to screen high risk youth for prediabetes and/or type 2 diabetes mellitus. Ann Pediatr Endocrinol Metab 2019;24:71-7. [Crossref] [PubMed]
- Hackshaw A. Small studies: strengths and limitations. Eur Respir J 2008;32:1141-3. [Crossref] [PubMed]
- Pietrzykowski T, Smilowska K. The reality of informed consent: empirical studies on patient comprehension-systematic review. Trials 2021;22:57. [Crossref] [PubMed]
- Waffenschmidt S, Knelangen M, Sieben W, et al. Single screening versus conventional double screening for study selection in systematic reviews: a methodological systematic review. BMC Med Res Methodol 2019;19:132. [Crossref] [PubMed]
- Goodhue DL, Thompson RL. Task-Technology Fit and Individual Performance. MIS Q 1995;19:213-36. [Crossref]
- Sheng CS, Zeng WF, Huang QF, et al. Accuracy of a Novel Non-Invasive technology based EZSCAN system for the diagnosis of diabetes mellitus in Chinese. Diabetol Metab Syndr 2011;3:36. [Crossref] [PubMed]
- Sai Archana P, Saraswathi Gopal K, Harsha Vardhan BG, et al. Saliva as a Non-invasive Tool in Evaluation of Type 2 Diabetes Mellitus. Int J Sci Study 2016;4:178-82.
- Sivanandam S, Anburajan M, Venkatraman B, et al. Estimation of blood glucose by non-invasive infrared thermography for diagnosis of type 2 diabetes: an alternative for blood sample extraction. Mol Cell Endocrinol 2013;367:57-63. [Crossref] [PubMed]
- Malik S, Parikh H, Shah N, et al. Non-invasive platform to estimate fasting blood glucose levels from salivary electrochemical parameters. Healthc Technol Lett 2019;6:87-91. [Crossref] [PubMed]
- Lin T, Mayzel Y, Bahartan K. The accuracy of a non-invasive glucose monitoring device does not depend on clinical characteristics of people with type 2 diabetes mellitus. J Drug Assess 2018;7:1-7. [Crossref] [PubMed]
- Takemoto I, Kawagoe N, Kijima S, et al. (13)C-glucose breath tests: a non-invasive method for detecting early clinical manifestations of exogenous glucose metabolism in type 2 diabetic patients. Acta Diabetol 2019;56:449-56. [Crossref] [PubMed]
- Tiongco RE, Bituin A, Arceo E, et al. Salivary glucose as a non-invasive biomarker of type 2 diabetes mellitus. J Clin Exp Dent 2018;10:e902-7. [Crossref] [PubMed]
- Tiongco REG, Arceo ES, Rivera NS, et al. Estimation of salivary glucose, amylase, calcium, and phosphorus among non-diabetics and diabetics: Potential identification of non-invasive diagnostic markers. Diabetes Metab Syndr 2019;13:2601-5. [Crossref] [PubMed]
- Tierney MJ, Tamada JA, Potts RO, et al. Clinical evaluation of the GlucoWatch biographer: a continual, non-invasive glucose monitor for patients with diabetes. Biosens Bioelectron 2001;16:621-9. [Crossref] [PubMed]
- Boer M, Duchnik E, Maleszka R, et al. Structural and biophysical characteristics of human skin in maintaining proper epidermal barrier function. Postepy Dermatol Alergol 2016;33:1-5. [Crossref] [PubMed]
- Kenny GP, Sigal RJ, McGinn R. Body temperature regulation in diabetes. Temperature (Austin) 2016;3:119-45. [Crossref] [PubMed]
- Wilson JMG, Jungner G. Principles and practice of screening for disease. Geneva: World Health Organization; 1968.
- Kirkman MS, Briscoe VJ, Clark N, et al. Diabetes in older adults. Diabetes Care 2012;35:2650-64. [Crossref] [PubMed]
- Siddiqui S, Zainal H, Harun SN, et al. Gender differences in the modifiable risk factors associated with the presence of prediabetes: A systematic review. Diabetes Metab Syndr 2020;14:1243-52. [Crossref] [PubMed]
- Gerdes M, Trinugroho YBD, Næss M, et al. Security, Reliability and Usability of mHealth Environments. Mobile Health 2015. doi:
10.1007/978-3-319-12817-7_43 .10.1007/978-3-319-12817-7_43 - Standards of medical care for patients with diabetes mellitus. Diabetes Care 2003;26:S33-50. [Crossref] [PubMed]
- Kharroubi AT, Darwish HM. Diabetes mellitus: The epidemic of the century. World J Diabetes 2015;6:850-67. [Crossref] [PubMed]
- Misra A, Gopalan H, Jayawardena R, et al. Diabetes in developing countries. J Diabetes 2019;11:522-39. [Crossref] [PubMed]
- Tang L, Chang SJ, Chen CJ, et al. Non-Invasive Blood Glucose Monitoring Technology: A Review. Sensors (Basel) 2020;20:6925. [Crossref] [PubMed]
- Xue Y, Thalmayer AS, Zeising S, et al. Commercial and Scientific Solutions for Blood Glucose Monitoring-A Review. Sensors (Basel) 2022;22:425. [Crossref] [PubMed]
- Davison NB, Gaffney CJ, Kerns JG, et al. Recent Progress and Perspectives on Non-Invasive Glucose Sensors. Diabetology 2022;3:56-71. [Crossref]
- Hilliard ME, Levy W, Anderson BJ, et al. Benefits and Barriers of Continuous Glucose Monitoring in Young Children with Type 1 Diabetes. Diabetes Technol Ther 2019;21:493-8. [Crossref] [PubMed]
- Sheikh NK, Dua A. Iontophoresis Analgesic Medications. Treasure Island (FL): StatPearls Publishing; 2023.
- Moyano DB, Paraiso DA, González-Lezcano RA. Possible Effects on Health of Ultrasound Exposure, Risk Factors in the Work Environment and Occupational Safety Review. Healthcare (Basel) 2022;10:423. [Crossref] [PubMed]
- Lv H, Liu J, Zhen C, et al. Magnetic fields as a potential therapy for diabetic wounds based on animal experiments and clinical trials. Cell Prolif 2021;54:e12982. [Crossref] [PubMed]
- Dearing JW, Cox JG. Diffusion Of Innovations Theory, Principles, And Practice. Health Aff (Millwood) 2018;37:183-90. [Crossref] [PubMed]
- Kwak JH. A study on the evolution of post-smartphone technologies in the 5G technology environment. KSII Transactions on Internet and Information Systems 2020; [Crossref]
- Moses JC, Adibi S, Wickramasinghe N, et al. Smartphone as a Disease Screening Tool: A Systematic Review. Sensors (Basel) 2022;22:3787. [Crossref] [PubMed]
- Moses JC, Adibi S, Angelova M, et al. Smart Home Technology Solutions for Cardiovascular Diseases: A Systematic Review. Appl Syst Innov 2022;5:51. [Crossref]
- DataBank. World Development Indicators 2020 [cited 2020 20 September]. Available online: https://databank.worldbank.org/reports.aspx?source=2&series=IT.CEL.SETS.P2
- Li D. 5G and intelligence medicine-how the next generation of wireless technology will reconstruct healthcare? Precis Clin Med 2019;2:205-8. [Crossref] [PubMed]
- Ericsson. Ericsson Mobility Report [Internet]. 2020. Available online: https://www.ericsson.com/en/reports-and-papers/mobility-report
- Morak J, Schreier G. Design and Evaluation of Near Field Communication (NFC) Technology Based Solutions for mHealth Challenges. Mobile Health 2015. doi:
10.1007/978-3-319-12817-7_35 .10.1007/978-3-319-12817-7_35 - Cheung NW, Redfern J, Thiagalingam A, et al. Text messaging support for patients with diabetes or coronary artery disease (SupportMe): protocol for a pragmatic randomised controlled trial. BMJ Open 2019;9:e025923. [Crossref] [PubMed]
- Dening J, Islam SMS, George E, et al. Web-Based Interventions for Dietary Behavior in Adults With Type 2 Diabetes: Systematic Review of Randomized Controlled Trials. J Med Internet Res 2020;22:e16437. [Crossref] [PubMed]
- Islam SMS, Mishra V, Siddiqui MU, et al. Smartphone Apps for Diabetes Medication Adherence: Systematic Review. JMIR Diabetes 2022;7:e33264. [Crossref] [PubMed]
- Dash S, Shakyawar SK, Sharma M, et al. Big data in healthcare: management, analysis and future prospects. J Big Data 2019;6:54. [Crossref]
- Madanian S, Parry DT, Airehrour D, et al. mHealth and big-data integration: promises for healthcare system in India. BMJ Health Care Inform 2019;26:e100071. [Crossref] [PubMed]
- Pastorino R, De Vito C, Migliara G, et al. Benefits and challenges of Big Data in healthcare: an overview of the European initiatives. Eur J Public Health 2019;29:23-7. [Crossref] [PubMed]
Cite this article as: Moses JC, Adibi S, Wickramasinghe N, Nguyen L, Angelova M, Islam SMS. Non-invasive blood glucose monitoring technology in diabetes management: review. mHealth 2024;10:9.