
Walk a mile in my shoes: perspectives towards sharing of health and experience data among individuals living with sickle cell disorder
Highlight box
Key findings
• Those living with the rare condition sickle cell disorder were supportive of collecting/sharing personal health data with healthcare professionals (HCPs), charitable organisations and pharmaceutical companies; to improve understanding of day-to-day condition impact.
• This would be more informative if focusing on outcomes patients associate with “feeling well” day-to-day, which often differ from the data needs of HCPs.
What is known and what is new?
• The willingness of those living with rare conditions to share personal health data is well reported. However, no prior study has highlighted the perceived utility of sharing patient-defined data concerning day-to-day variability; nor have previous reports of willingness-to-share personal health data with pharmaceutical organisations been as high as those observed in this study.
What is the implication, and what should change now?
• As with COVID-19, digital health technologies will be an increasingly valuable tool in improving understanding of poorly understood health conditions, and particularly the day-to-day impact. But consultation with patients is critical, as not all data types are considered of equal value, benefit, or risk.
Introduction
People living with long-term conditions are increasingly using digital health technologies (DHTs) to record and monitor health data, using it to manage their personal health (1-3); encompassing a broad range of information such as medication adherence, health and lifestyle practices, outcomes and experiences. Advancements in these DHTs have arguably made this process simpler, with numerous datapoints passively recorded and analysed, providing the potential to improve both the management of long-term health conditions (4) and resulting quality-of-life.
While the benefits of collecting real-world data (RWD) about one’s own health may be numerous, including tailored support from healthcare professionals (HCPs) (5,6) and peer-support communities alike (7); the wider scale collection, curation and analysis of personal health data may provide unrivalled opportunities in advancing current understanding of the natural history and progression of health conditions that may otherwise remain poorly understood (8,9). Furthermore, the COVID-19 pandemic may have inadvertently acted as a catalyst for the systematic coordinated sharing of routinely collected health data, with 13,395 publications of real-world evidence on COVID-19, concerning epidemiology, quality-of-life, and symptoms in just two years since the beginning of the pandemic (9).
It is this ability, to collect, curate and interrogate digitally derived health data which is of particular interest within rare disorders, a group of more than 10,000 conditions, affecting approximately 350,000,000 people worldwide (10). Exceptionally little is known about the day-to-day impact of these conditions (11), how they affect quality-of-life, symptoms, treatment side effects, and importantly, the unmet needs individuals living with rare disorders face. In this regard, our understanding of rare disorders is not dissimilar to our understanding of COVID-19 early in the pandemic. Approximately 79% of UK-based general practitioners (GPs) are unsure how to manage patients with rare disorders (12), with this lack of understanding likely impacted by several difficulties in coordinating and conducting experimental research, including patient identification, unclear definitions of patient outcomes, and significant heterogeneity (13,14).
Consequently, regulatory authorities are increasingly recognising the value of RWD often derived via digital health (15), to inform both pre- and post-authorisation regulatory decision making for rare disorder therapeutics (16-18). However, with patients not just the recipients of care, but rather experts with lived experience of these largely misunderstood conditions, the potential exists to expand usage of digitally derived RWD to capture outcomes of most relevance to patients, moving beyond “hard endpoints” (19) to include patient experience, and patient-reported experience measures (PREMs) (19), with a more focused and holistic appreciation of patients’ most critical needs, desires and aspirations.
While many prior studies have explored the barriers and facilitators of sharing electronic health record (EHR), and registry data (20,21), few have explored what “feeling well looks like” to patients with rare disorders and how this can be captured via digitally derived RWD. The aim of this mixed-methods study, conducted with individuals living with a group of rare haematological conditions [sickle cell disorder (SCD)] was to therefore explore (I) what ‘feeling well’ looks like for someone living with SCD; (II) how this could be best monitored and reported day-to-day using DHTs; (III) which data patients believe HCPs should pay more attention to and; (IV) which types of data individuals affected by SCD would be willing to share, with whom, and under what conditions.
Methods
Participants & eligibility
Utilising a mixed-methods design, a semi-structured focus group was initially conducted to explore patient perspectives and experiences, with identified key themes further tested with a wider audience using a larger scale quantitative survey. For the focus group, individuals aged 18 and over with lived experience of SCD and who had provided informed consent to take part in the study, were recruited on a voluntary basis. We focused on SCD, as this is a rare condition subject to considerable day-to-day variability, with several key considerations impacting quality-of-life, not all of which being routinely observed within the health record, including hydration, the onset of sickle cell crises, infection, swelling, side effects from medication, including opiates, and issues with visual acuity. Following introductions by a UK-based national sickle cell charity (the Sickle Cell Society), an advertisement poster was co-designed by individuals living with SCD and disseminated on the social media pages (LinkedIn and Twitter) of the Sickle Cell Society.
For the survey, individuals aged 18 and over with lived experience of SCD self-selected to complete the survey. While no formal verification of SCD status was performed, the study was advertised both via a closed private SCD Facebook group and via a verified sickle cell patient support group (the Sickle Cell Society). A copy of the advertisement shared on the Facebook group is provided in Figure S1.
In recognition of their time and expertise, both focus group and survey participants received a £15 Amazon voucher or could request a charitable donation for the same monetary value. While remunerating participants for their time may have biased their motivations for taking part, recognising people’s time and expertise is the best practice in co-design the patient and public involvement research (22-24).
Procedures (focus group)
The focus group analysis centred on one primary question “What does ‘feeling well’ look like for someone with SCD and how could this be best monitored and reported using digital health technologies day-to-day?” We also asked a series of secondary, related questions including (I) which, if any, data do you feel that HCPs should pay more attention to when supporting you in managing your SCD? and (II) which types of data, if any, would you be willing to share, with whom, and under which conditions?
To address these core questions, a semi-structured topic guide informed by existing literature (25) was co-designed with a patient research partner (Z.G.S.) who the authors engaged with via the Sickle Cell Society. Topic guide questions were purposefully open-ended to facilitate in-depth discussions about patient perceptions and experiences of data sharing with suggested prompts also provided to further facilitate discussion/clarification if required. Due to ongoing COVID-19 concerns and restrictions, the focus group was held online via Zoom. The focus group was jointly facilitated by an experienced qualitative researcher and the patient research partner with combined expertise in qualitative research methods and lived experience of SCD. The focus group was audio recorded, conducted with informed consent, transcribed verbatim and anonymised independently by two members of the research team.
Procedures (survey)
Based on the findings of the focus group, the cross-sectional survey was developed in line with the Checklist for Reporting Results of Internet E-Surveys (CHERRIES) reporting standards (26). As such, the survey examined attitudes towards what patients believed ‘feeling well’ looks like for someone with SCD, and attitudes towards sharing this data. The survey also explored if and how respondents had previously shared their health data, whilst gauging prior beliefs regarding the utility and benefit of doing so. A copy of the survey is provided in Appendix 1.
Prior to being offered to participants, the survey was tested for consistency by two members of the research team, the patient partner and subsequently piloted among a small number of people with SCD to assess usability, readability and interpretation. Recruitment for the pilot phase of the survey was conducted over three working days in July 2022, with recruitment for the finalised survey conducted over one week in September 2022. As with numerous prior studies, the survey was administered via Google Forms.
Given the nature of SCD, a group of rare haematological disorders, we aimed for a realistic sample of 50 people from any location to explore, validate, or refute the findings of the prior focus group which was focused predominantly on UK perspectives to health data sharing. The survey remained open until the required sample of participants was reached. The survey was offered in English language only due to resource constraints, and participants were informed approximately how long the survey would take. No personally identifiable data were collected or stored, and all participants were reimbursed for their participation in the survey with a £15 Amazon voucher. Additionally, each respondent was given entry to a draw to win either a £100 or £40 Amazon voucher. Given the short duration required to complete the survey (approximately 5 to 10 minutes), the order in which questions appeared was not randomised as we anticipated the likelihood of survey fatigue to be minimal in such a short survey.
Data integrity checks and mechanisms to protect against unauthorised access were built into the study design, including only advertising via private and closed Facebook groups and through direct contact via patient support groups. While IP checks were not performed, we used the Google Forms feature limiting responses to one per person per email address. Additionally, frequency checks were performed once responses were submitted in order to identify suspicious submission patterns, including surveys being completed once every ~10 minutes for a sustained and continuous period of time. Finally, participants could not amend, review or change their answers to a question once submitted, with no back button and no summary page of responses provided.
Measures
Survey questions were co-developed in partnership between the research team and the patient research partner, a copy of which is provided in Appendix 1. In addition to primary questions concerning (I) what ‘feeling well’ looks like for someone with SCD; (II) how this could be best monitored and reported day-to-day; (III) which, if any, data respondents believed HCPs should pay more attention to; and (IV) which, if any, types of data respondents would be willing to share, with whom, and under which conditions, we additionally collected responses concerning SCD activity, including the number of sickle cell crises and hospitalisations respondents had recently experienced; in addition to basic demographic characteristics of respondents including gender, age and ethnicity, on account of acknowledged differences in data sharing attitudes across gender, age and ethnicity (7,20,25).
Statistical analysis
We imported participant responses into Microsoft® ExcelTM (Redmond, WA, USA). We present summary statistics to describe the characteristics of the participants. Categorical variables were summarised by frequency and percentage, with continuous variables reported as mean and standard deviation (SD). Subgroup analyses, segmented by markers of condition activity and demographics (age), were also conducted. For comparison of attitudes, barriers and motivators for data sharing between sub-groups, all statistical analyses were conducted using STATA 14 (StataCorp LP, USA).
Patient and public involvement
The study design, focus group topic guide and specific survey questions were co-designed with a patient research partner living with lived experience of SCD.
Ethics
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study obtained ethical approval from the University of Plymouth Faculty Research Ethics and Integrity Committee (Approval No. 2826). All participants in both the focus group and survey provided informed consent.
Results
Focus group
In response to the primary research question of “What does feeling well look like for someone with SCD and how could this be best monitored and reported?”, focus group participants (n=25) identified a range of measures including mindset, service utilisation and lifestyle choices they used to assess and monitor their condition management (Table 1).
Table 1
| Participant defined measures of condition management | Verbatim examples |
|---|---|
| Number of hospital visits | “The way I used to count it was the number of days where I didn’t have a hospitalisation. And I thought that was a very toxic way of trying to measure how well I am because there’ll be times where I have a really bad crisis, but because I’m having like a streak of however many months, I got up to just over two years once, that will kind of stop me from going and getting the help I need” (participant 1) |
| Associated complications | “It might not be the pain, but I have gallstones, or my eyes are being impacted, something’s going on in my body related to sickle cell and there’s something that I can’t ignore” (participant 5) |
| Lifestyle choices and healthy habits | “Using vitamins, exercising etc. Just general healthy habits which tend to help you have a better of quality of life … just how well I manage to do all of those (healthy lifestyle choices and habits) as opposed to actually just avoiding certain things” (participant 3) |
| “Eating a balanced diet” (participant 6) | |
| “Drinking lots of water” (participant 5) | |
| Sociability | “How social I’m able to be … I’m a huge extrovert and I get my energy from other people so normally I would love to have a full calendar of social events. But if I start getting really anxious about that or saying I’m just too tired and I feel like I’ve been putting them off, I feel that’s my sickle cell talking saying you actually just don’t have the energy at the moment and you have to use that energy to look after your health” (participant 2) |
| Mindset | “A positive mindset” (participant 8) |
| “It’s difficult, of course it’s difficult. We’ve all got the mindset that we know that sickle cell is in the background but how I deal with it is I’m like ‘no I’m going to conquer this. I have to conquer this” (participant 4) | |
| Mental health/emotional wellbeing | “The reason why I said feelings is because a lot of the time we think more of a physical pain when it comes to sickle cell, but mentally, it’s extremely detrimental. The pain is what it is. But mentally, it can be a real downer” (participant 4) |
| “Sometimes it’s not about you know, what’s actually happening within you as in, how’s the kidney stones going? How’s the vascular necrosis? Sometimes it’s just, how have you actually coped with living? How have you coped with waking up and going to work? How have you coped with waking up and not going to work? The mental side of sickle cell always gets left out, having a chronic illness and living with it every day, no matter how strong a person you are, no matter how positive you are, it is going to have an effect and you will have days where you really have to lift yourself up … I mean, how often are you asked that question, how often do people actually ask, ‘how are you feeling? Not pain wise, how are you feeling in yourself?’ That’s a big one and the one that’s tackled the least” (participant 3) |
Measures of condition management described by participants often focused on “everything else that comes with” SCD as opposed to predominantly clinical measures typically collected in EHRs, with many participants suggesting valued patient measures are currently missing from existing data collection methods and tools.
“It’s not about, you know, what’s actually happening within you. As in how’s the kidney stones going? How’s the vascular necrosis? Have you had any crisis? Sometimes it’s just how have you actually coped with living? How have you coped with waking up and going into work? If you are going to work? How have you coped with not waking up and going to work? And sickle cell, the mental side of sickle cell always gets left out, having a chronic illness and living with it every day, no matter how strong a person you are, no matter how positive you are, it is going to have an effect and it will have days where you really have to lift yourself up.” (participant 3).
Perceptions of how desired measures of condition management could be best monitored and reported included measuring sickle cell “day to day” (participant 6). The value of measuring condition management on such a regular basis was often juxtaposed against the significant delay between consultant/specialist visits. For example, “I see my consultant every six months. So within those months I have to manage myself, my medication and everything else that comes with it.” (participant 4).
This “day-to-day” perspective on the impact of SCD was contrasted with the rather fixed and one-off nature of EHRs, which tended to collect data usually at pre-determined snapshots of time, and often may not cover outcomes which those living with SCD believed were associated with feeling well. The perceptions of the focus group participants regarding data sharing are presented in conjunction with the results from the online survey below.
Survey
Demographics & baseline characteristics
Fifty adults responded to the survey with two failing to meet the inclusion criteria and one failing to answer the questions provided, resulting in a 94% completion rate. Demographics and baseline characteristics of the survey respondents are provided in Table S1.
Defining “what good looks like”, and the types of data participants would be willing to be share
When asked on a scale from 0 (not willing to share at all), to 10 (very happy to share), survey respondents were typically willing to share all suggested data types (Figure 1). Respondents were most willing to share demographic data (8.55/10), symptoms related to their condition (8.45/10) and data concerning health service utilisation (8.23/10). Respondents were least likely to share genomic (7.66/10) and health record data (7.3/10). Similar notions were also reflected in the focus group. For example, “I worked in genomics so I realise some of the impact that you can have if you share your genomic data, like insurance and all that kind of stuff. So I feel like I was asking myself more questions and I was more weary of it.” (participant 2). Similarly, “I think when it comes to sharing my details, like as my actual hospital records, you know, really in depth and GP, no, I would I think I would be more what do you need it for? What are you going to do with it? But I think actual experience personal experience, I have no problem at all.” (participant 3). However, similar to the quantitative results presented, some participants were willing to share such data reflecting the importance of choice and voice in what data is shared and by whom:
When asked the secondary research questions of which, if any data should HCPs pay more attention to, 70.2% of respondents believed that HCPs should pay more attention to how “I am feeling mentally”, with 63.8% and 61.7% reporting they believed HCPs did not pay enough attention to their lifestyle (diet and exercise), and daily fluctuations in symptoms, mental and physical health respectively. The importance of mental health/emotional wellbeing was repeatedly reiterated by focus group participants as shown in Table 1.
Factors influencing the decision to share health data (including motivations/incentives)
When asked on a scale from 0 (completely disagree) to 10 (completely agree), respondents generally agreed that sharing their health data may benefit other people with SCD (7.94/10). Conditions found to facilitate respondents’ willingness to share health data included trust in the organisations seeking data access and use, transparency in data sharing use and financial incentives.
Trust in the organisation seeking and using respondents’ data was the single largest facilitator of health data sharing, reported by 72.3% of respondents, while 61.7% of respondents stated that knowing how their data will be used would influence their decision. Similar notions were also reported in the focus group. For example, “straight away, if I don’t feel comfortable and I feel like there’s not 100% honesty then I’m not sharing it. But once I’m told what it entails and what it’s going to be used for, then I usually share it.” (participant 3). Participants also appeared to value, although to a lesser extent, the ability to withdraw consent for using health data at any point (49%), knowing data was stored in a secure facility (42.6%) and knowing that organisations which misuse data will be subject to large fines or penalties (36.2%).
Other conditions found to facilitate data sharing included personal incentives for actively sharing health data. Financial benefits (or shopping vouchers) in exchange for the time taken to provide health-related data were the most commonly reported factor influencing the decision to share data (74.5%), followed by knowing how your data has helped others (48.9%) and knowing that friends/peers were also sharing their data (44.7%), as demonstrated in Table 2.
Table 2
| Factors | Percentage (%) |
|---|---|
| Financial benefits (e.g. cash payments shopping or Amazon vouchers, etc.) | 74.5 |
| Knowing how your data has helped other people with sickle cell disorder | 48.9 |
| Knowing that my friends/peers are also sharing their health data | 44.7 |
| Knowing precisely how your data is going to be used | 42.6 |
| Being made aware of new treatments or trials which you may be eligible for | 42.6 |
| Personalised guidance and insights about your symptoms and condition management | 40.4 |
| Financial donations to charities (including patient support groups) | 40.4 |
| Being made aware of your contribution to sickle cell disorder research | 40.4 |
Previous sharing of health-related data
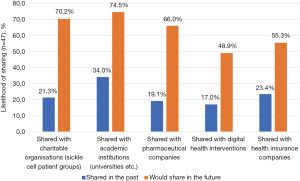
While 44.7% of respondents had previously shared some form of health data, the organisations with whom they had shared this data with varied (Figure 2), with academic institutions (34.0%), health insurance companies (23.4%) and charitable organisations (21.3%) most commonly represented. Across all organisational types, the proportion of respondents willing to share data was consistently greater than the proportion who had previously shared data with these organisation types. The largest opportunity reported by respondents was within the pharmaceutical industry where 19.1% of respondents had previously shared data, and 66.0% were willing to do so in the future; suggesting that 71% of those willing to share health data were yet to do so. This was followed by charitable organisations where 70% of those willing to share data were yet to do so (21.3% shared to date compared to 70.2% willing to share) and digital health interventions (65.2%).
Discussion
Principal findings
Responding to gaps in existing literature (12,13,19), this research explores what ‘feeling well’ looks like for those living with SCD, how this could be best monitored and reported day-to-day using DHTs, and the barriers and facilitators to sharing this data for the purposes of improved condition understanding and research. Measures of condition management considered to be of greatest importance from a patient perspective were often focused on “everything else that comes with” their condition. Central to this was “measuring sickle cell on that day-to-day basis”, with most participants believing that healthcare providers did not pay enough attention to day-to-day variability in symptoms and outcomes. A willingness for frequent recording and reporting was often juxtaposed against the significant delay between consultant/specialist visits. Patients were willing to share symptom data, and those concerning health service utilisation, but were less eager to share genomic and health record data. However, conditions found to facilitate patients’ willingness to share health data included personal incentives for actively sharing health data, and being informed about how their data had helped others. Finally, the largest gaps between willingness and previous sharing of health data were for the pharmaceutical industry, charitable organisations and digital health interventions, where 71%, 70% and 65.2% respectively, of those willing to share health data, were yet to do so suggesting a current disparity between patient willingness to share health data and actioned behaviour. As discussed, this may be due to factors shown to influence patient willingness including trust, transparency and relevant incentives not yet being fully realised from a patient perspective. The onus is therefore not on patients to just ‘share’ their data, but rather for organisations and DHTs to create a safe and appealing environment where patients feel comfortable and empowered in sharing their health data.
Interpretation
Prior research has shown that those living with rare disorders, regardless of the severity of their condition and their socio-demographic profile, are supportive of data sharing to foster research and improve healthcare (7), with DHTs increasingly being used to expedite this process and inform how such data collected via digital health can be better used to improve patient outcomes and experiences (19).
Our findings suggest that the measures of condition management of most importance to patients were not the hard clinical and laboratory-based measures typically used to define condition management, but instead focused on “everything else that comes with” their condition. Key to this was “measuring sickle cell on that day-to-day basis”, which was often juxtaposed against the significant delay between consultant/specialist visits. This finding therefore suggests a disparity between what is currently being measured and therefore valued by clinicians in condition management, and what is valued by “empowered” patients (27,28). This suggests a key role for such data in filling gaps in our understanding of poorly understood conditions from the patients’ unique perspective, as opposed to simply providing “biocapital” for others (29,30), including science and technology industries to harness and exploit, as suggested elsewhere (31).
This finding aligns with the recent movement among health regulators to not only focus on “hard endpoints” (17-19) but also on patient experience. Most participants in our study believed that healthcare providers did not pay enough attention to day-to-day variability in both symptoms and outcomes, with 70.2% of respondents believing HCPs should pay more attention to how “I am feeling mentally”, with 63.8% and 61.7% reporting they believed HCPs did not pay enough attention to their lifestyle (diet and exercise). This may be a result of a lack of awareness among HCPs in knowing what matters to patients with specific rare disorders. Recent studies of primary and secondary care providers in Kazakhstan (32), the United Kingdom (12) and Poland (33) have demonstrated that most physicians lacked basic knowledge about the aetiology, epidemiology and prevalence of rare conditions, with 94.6% of physicians perceiving their knowledge on rare disorders as insufficient or very poor, and less than 5.3% feeling prepared for caring for patients with rare disorders (33). Co-design between patients and HCPs may help broaden current understanding and better align these stakeholder perspectives and expectations with regard to data collection and sharing. This requirement to better understand the experiences of patients and how digital health may play a role in broadening understanding has been well documented among lower-income or culturally marginalized individuals (7). This research therefore provides a valuable addition to the literature, particularly in light of the struggles those living with SCD face in being heard (34-36).
As suggested in prior research (7), understanding what patients want and need from rare disorder research and data sharing, is critical to ensuring their participation and engagement in the process as not all data types are considered of equal benefit, or of equal risk. We found that patients were willing to share symptom data, and those concerning health service utilisation, but were far less willing to share genomic and health record data. This is a finding corroborated in prior research which showed that genetic information is of particular concern among patients. A recent study highlighted that 35% of respondents believed genetic information is very sensitive (7), while wider research shows a similar trend of genomic data being considered more ‘sensitive’ than others (37).
A hesitation linked to sharing genomic and health record data is often linked to a fear of reprisals, namely data being used against individual patients or collective communities (38-43) including a reduction or rejection in medical insurance cover (35,40,44-48) and employment opportunities (40,46,49-50). Prior studies have shown that willingness to share health data among those with rare disorders comes with specific requirements in order to respect their privacy, choices and needs for information regarding the use of their data (7), with such conditions viewed as safeguards preventing data from being misused, surreptitiously extracted or used to serve agendas that benefit research and industry as opposed to patients (51,52).
In fact, emerging research suggests patients may be willing to share more sensitive types of data such as genomic data, provided explicit permission has been sought and policies reflect patient expectations for how this should be achieved (52,53). Confidence and willingness to share more sensitive data is therefore not unachievable. For example, a 2018 study exploring the reasons why those living with rare disorders shared their personal health data online found that participants had several concerns about privacy, but that the motivation for sharing despite this risk was that it could lead to new developments, thereby helping themselves and others (53). Another study (54) found similar results, that patients (in their case members of a European leukodystrophies database) supported data sharing in order to generate greater knowledge and clinical outcomes. While different demographic groups may have different responses and attitudes towards data sovereignty, prior research does suggest that patients living with a rare disorder are increasingly willing to engage with research, as it often offers the only hope of accessing a diagnosis or benefitting from a treatment or a cure. Linked to this theme of facilitators of data sharing, we found that conditions promoting patients’ willingness to share health data included trust in the organisations seeking data access and use, transparency in data sharing and use, provision of personal incentives, and knowing how your data has helped others.
However, the willingness of patients and their families to support the scientific research agenda and engage with biomedical research and data sharing can often leave them vulnerable (7). One of the key problems with research into rare disorders has been that the hope and promises associated with developments in technologies have often been slow to translate into clinical outcomes, and that while there might be scientific merit, patient communities have often not experienced any benefit. This links to our finding of providing more immediate and personal incentives for participation, particularly if companies are known to be making commercial gains from data sharing practices (55-57). While for many, the incentive of more altruistic benefits is enough (56,58), a range of incentives including refunds or compensation from health insurers (59), financial incentives (57,59) availability of research results (23,60) may provide sufficient incentives to take part in rare disorder research and data sharing (61).
It is worth noting that our findings differ to the majority of research that says data security and management is often the most significant influencing factor in affecting people’s willingness to share data. In some studies, data privacy was the most significant concern in comparison to other factors reviewed (23,50,61,62) while in our survey the importance of data privacy, while high, was less pronounced compared to other factors. Having the ability to withdraw consent for using health data at any point was a facilitator of data sharing among 49% of respondents, while knowing data was stored in a secure facility (42.6%) and knowing that organisations which misuse data will be subject to large fines or penalties (36.2%) were also important, as also reported elsewhere (40).
While our finding that respondents were more likely to share personal health data with academic and public institutions is not a novel finding, with trust in not-for-profit stakeholders well documented to be much higher than for-profit stakeholders (63), our finding that the greatest “untapped” opportunity for data sharing is with for-profit stakeholders is a novel one. Across all organisational types, we found that the proportion of respondents willing to share data was consistently greater than the proportion who had previously shared data with these organisation types, across all for-profit and not-for-profit stakeholders. However, the largest opportunity reported by respondents was within the pharmaceutical industry where 71% of those willing to share health data were yet to do so, followed by charitable organisations (70%) and digital health interventions (65.2%). This is opposed to previous literature that has historically suggested low support for pharmaceutical use (54,63,64). While previous research has often focused on a single population with just one of many rare disorders, this general theme may be reflective of a change in attitudes towards data sharing post COVID-19. While yet to be assessed for validity and generalisability in other rare disorders, our research highlights the importance of regularly reviewing patient perspectives regarding DHTs and data sharing practices, given the variable and changeable nature of technology, condition awareness and data sharing practices.
Strengths and limitations
While several prior studies have focused on approaches to genetic data pooling and registries sharing data (65,66), there has until now been an absence of published evidence dedicated to attitudes concerning the sharing of patient-level health and experience data in rare disorders. This study therefore provides a novel addition, by going beyond data sharing concerning the EHR and the more clinically focused patient registries, instead focusing on real-world symptom and experience data which may be more readily harnessed via the use of digital and other health technologies. Additionally, the study being co-designed and coordinated by those living with a rare disorder, maximised relevance, highlighting what those living with SCD truly believe to be the information that healthcare providers, should listen to, but are not currently listening to, presenting a role for future data collection and sharing.
However, there are also several limitations to this analysis which may impact the generalisability of the findings. The first and most obvious limitation is that this analysis concerned only a small group of people living with one specific rare disorder, SCD, which in itself is a highly heterogeneous group of conditions. As such, it would be beneficial to repeat this exercise with other, more diverse and representative groups of people living with a variety of rare disorders, including family members and caregivers whose views were also absent from this study; in order to confirm the generalisability of the findings beyond SCD.
This links to a further limitation of the study was that despite the focus group being quite large from a qualitative perspective, the size of the survey was quite restrictive. Given the number of participants, it is entirely likely that the ability to participate was reduced and therefore that some opinions were not, or could not be expressed, a limitation of using a quota approach to sampling. Furthermore, the nature of recruitment meant that it is likely only those with a specific position for or against data sharing were likely to attend, therefore disproportionately attracting more fixed or even extreme views. This may have been further compounded by advertising both the focus group and survey via social media, and requiring respondents to take part digitally. We had a high number of respondents aged 35 or under, and the age breakdown of those involved in our study may not be reflective of the population norms for SCD. Added to this is the limitation that we did not collect demographic data as part of the initial focus group, which informed the latter survey. Therefore, we cannot be certain that we achieved a balanced mix of views from those of varying ages, ethnicities and socio-economic status, which may have subsequently impacted our ability to delve into certain themes, and prevented exploration of these themes during the subsequent survey. Additionally, the availability of the survey in the English language only may have also led to a more limited sample as this may be exclusionary to certain Black, Asian and Minority Ethnic (BAME) populations, which would be expected to be highly prevalent among those living with SCD, a condition which predominantly affects BAME populations. While recruitment among those living with rare disorders is generally more problematic than other condition areas (67), a larger sample and alternative means for inclusion, including face-to-face focus groups and paper surveys, would have enabled the attitudes of the median person with SCD to be highlighted, and the findings more likely to be generalisable to wider populations. Further research should mirror this work in other rare disorder cohorts to determine if the findings are generalisable, and if in fact, patients are willing to systematically collect and share health and experience data through with the assistance of DHTs.
Future research
Future research should examine how the findings presented here can be put into practice. Respondents in both our focus group and survey were clear in their belief that more should and can be done to understand the full spectrum of what it is like to live with a rare condition, and that digital health can play a valuable role in filling this gap. What is unclear is how such a technology would need to look, the features required, and how feasible collection of such data is on a routine basis. Key to this is considering not only willingness to provide data, but also the quality of such data collected in real-world “non-controlled” settings, and how influential this is for healthcare providers required to make decisions regarding their patients’ care.
Conclusions
Patients living with SCD, a rare condition which is characterised by poor understanding and awareness, were supportive of data sharing to foster research and improve healthcare. However, patients had specific requirements in order to respect their privacy, choices and needs for information regarding the use of their data. Measures of condition management of most importance to patients often focused on “everything else that comes with” their condition, which they believed HCPs did not pay enough attention to, including day-to-day variability in symptoms and outcomes. Understanding what patients want and need from rare disorder research and data sharing is critical to ensuring their meaningful involvement in the process as not all data types were considered of equal benefit or risk.
Acknowledgments
The authors would like to take the opportunity to thank those who gave up their time and shared their valuable expertise by taking part in this study.
Funding: The research was funded by a small business innovation grant as part of the eHealth Productivity and Innovation in Cornwall and The Isles of Scilly (EPIC) Programme, a European Union Regional Development (ERDF) programme led by the University of Plymouth.
Footnote
Data Sharing Statement: Available at https://mhealth.amegroups.com/article/view/10.21037/mhealth-23-18/dss
Peer Review File: Available at https://mhealth.amegroups.com/article/view/10.21037/mhealth-23-18/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://mhealth.amegroups.com/article/view/10.21037/mhealth-23-18/coif). S.L., R.B. and S.S. report that the research was funded by a small business innovation grant as part of the eHealth Productivity and Innovation in Cornwall and The Isles of Scilly (EPIC) Programme, a European Union Regional Development (ERDF) programme led by the University of Plymouth. S.L. is the co-founder of Prometheus Health Technologies Ltd. R.B. is a partial share owner in Prometheus Health Technologies. Z.G.S. chairs the NHS England Patient Advisory Group for Sickle Cell Transformation and receives a patient involvement honorarium in line with NHS England policy. As part of this role, she is a member of related steering groups, committees and assessment panels; she has also conducted work as a paid consultant, performing research related activities for Prometheus Health Technologies, and is also a member of the Genomics England Diverse Data Advisory Group. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study obtained ethical approval from the University of Plymouth Faculty Research Ethics and Integrity Committee (Approval No. 2826). All participants in both the focus group and survey provided informed consent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lavallee DC, Lee JR, Austin E, et al. mHealth and patient generated health data: stakeholder perspectives on opportunities and barriers for transforming healthcare. Mhealth 2020;6:8. [Crossref] [PubMed]
- Jacoby SF, Robinson AJ, Webster JL, et al. The feasibility and acceptability of mobile health monitoring for real-time assessment of traumatic injury outcomes. Mhealth 2021;7:5. [Crossref] [PubMed]
- Shah SS, Gvozdanovic A, Knight M, et al. Mobile App-Based Remote Patient Monitoring in Acute Medical Conditions: Prospective Feasibility Study Exploring Digital Health Solutions on Clinical Workload During the COVID Crisis. JMIR Form Res 2021;5:e23190. [Crossref] [PubMed]
- Simpson E, Brown R, Sillence E, et al. Understanding the Barriers and Facilitators to Sharing Patient-Generated Health Data Using Digital Technology for People Living With Long-Term Health Conditions: A Narrative Review. Front Public Health 2021;9:641424. [Crossref] [PubMed]
- Sands DZ, Wald JS. Transforming health care delivery through consumer engagement, health data transparency, and patient-generated health information. Yearb Med Inform 2014;9:170-6. [PubMed]
- Cohen DJ, Keller SR, Hayes GR, et al. Integrating Patient-Generated Health Data Into Clinical Care Settings or Clinical Decision-Making: Lessons Learned From Project HealthDesign. JMIR Hum Factors 2016;3:e26. [Crossref] [PubMed]
- Courbier S, Dimond R, Bros-Facer V. Share and protect our health data: an evidence based approach to rare disease patients' perspectives on data sharing and data protection - quantitative survey and recommendations. Orphanet J Rare Dis 2019;14:175. [Crossref] [PubMed]
- Gamarel KE, Stephenson R, Hightow-Weidman L. Technology-driven methodologies to collect qualitative data among youth to inform HIV prevention and care interventions. Mhealth 2021;7:34. [Crossref] [PubMed]
- Dron L, Kalatharan V, Gupta A, et al. Data capture and sharing in the COVID-19 pandemic: a cause for concern. Lancet Digit Health 2022;4:e748-56. [Crossref] [PubMed]
- Fu MP, Merrill SM, Sharma M, et al. Rare diseases of epigenetic origin: Challenges and opportunities. Front Genet 2023;14:1113086. [Crossref] [PubMed]
- Plackowski EF, Bogart KR. "If not me, who?": Awareness- and Self-Advocacy-Related Experiences of Adults With Diverse Rare Disorders. Qual Health Res 2023;33:63-80. [Crossref] [PubMed]
- McMullan J, Crowe AL, McClenaghan T, et al. Perceptions and Experiences ofRare Diseases among General Practitioners: An Exploratory Study in NI. Arch Clin Med Case Rep 2023;7:248-56. [Crossref]
- Lopes MT, Koch VH, Sarrubbi-Junior V, et al. Difficulties in the diagnosis and treatment of rare diseases according to the perceptions of patients, relatives and health care professionals. Clinics (Sao Paulo) 2018;73:e68. [Crossref] [PubMed]
- Stoller JK. The Challenge of Rare Diseases. Chest 2018;153:1309-14. [Crossref] [PubMed]
- Demetri GD, Stacchiotti S. Contributions of Real-World Evidence and Real-World Data to Decision-Making in the Management of Soft Tissue Sarcomas. Oncology 2021;99:3-7. [Crossref] [PubMed]
- Eskola SM, Leufkens HGM, Bate A, et al. Use of Real-World Data and Evidence in Drug Development of Medicinal Products Centrally Authorized in Europe in 2018-2019. Clin Pharmacol Ther 2022;111:310-20. [Crossref] [PubMed]
- Mahendraratnam N, Mercon K, Gill M, et al. Understanding Use of Real-World Data and Real-World Evidence to Support Regulatory Decisions on Medical Product Effectiveness. Clin Pharmacol Ther 2022;111:150-4. [Crossref] [PubMed]
- Lau C, Jamali F, Loebenberg R. Health Canada Usage of Real World Evidence (RWE) in Regulatory Decision Making compared with FDA/EMA usage based on publicly available information. J Pharm Pharm Sci 2022;25:227-36. [Crossref] [PubMed]
- Wu J, Wang C, Toh S, et al. Use of real-world evidence in regulatory decisions for rare diseases in the United States-Current status and future directions. Pharmacoepidemiol Drug Saf 2020;29:1213-8. [Crossref] [PubMed]
- Papoutsi C, Reed JE, Marston C, et al. Patient and public views about the security and privacy of Electronic Health Records (EHRs) in the UK: results from a mixed methods study. BMC Med Inform Decis Mak 2015;15:86. [Crossref] [PubMed]
- Naeem I, Quan H, Singh S, et al. Factors Associated With Willingness to Share Health Information: Rapid Review. JMIR Hum Factors 2022;9:e20702. [Crossref] [PubMed]
- NIHR. Payment guidance for researchers and professionals. 2022. Available online: https://www.nihr.ac.uk/documents/payment-guidance-for-researchers-and-professionals/27392
- Baines R, Bradwell H, Edwards K, et al. Meaningful patient and public involvement in digital health innovation, implementation and evaluation: A systematic review. Health Expect 2022;25:1232-45. [Crossref] [PubMed]
- EFPIA. Working together with patient groups. 2017. Available online: https://www.efpia.eu/media/288492/working-together-with-patient-groups-23102017.pdf
- Adu EK, Mills A, Todorova N. Factors influencing individuals’ personal health information privacy concerns. A study in Ghana. Inf Technol Dev 2021;27:208-34. [Crossref]
- Eysenbach G. Improving the quality of Web surveys: the Checklist for Reporting Results of Internet E-Surveys (CHERRIES). J Med Internet Res 2004;6:e34. [Crossref] [PubMed]
- Topol EJ. The Creative Destruction of Medicine: How the Digital Revolution Will Create Better Health Care. New York: Basic Books; 2012:294-6.
- Lupton D. The Quantified Self: A Sociology of Self-Tracking. Cambridge, UK: Polity Press; 2016.
- Till C. Exercise as labour: Quantified Self and the transformation of exercise into labour. Societies 2014;4:446-62. [Crossref]
- Rabinow P, Rose N. Biopower today. BioSocieties 2006;1:195-217. [Crossref]
- Kahn J. Privatizing biomedical citizenship: risk, duty, and potential in the circle of pharmaceutical life. Minn J Law Sci Technol 2014;2014:15.
- Walkowiak D, Bokayeva K, Miraleyeva A, et al. The Awareness of Rare Diseases Among Medical Students and Practicing Physicians in the Republic of Kazakhstan. An Exploratory Study. Front Public Health 2022;10:872648. [Crossref] [PubMed]
- Walkowiak D, Domaradzki J. Are rare diseases overlooked by medical education? Awareness of rare diseases among physicians in Poland: an explanatory study. Orphanet J Rare Dis 2021;16:400. [Crossref] [PubMed]
- Andrews SM, Raspa M, Edwards A, et al. "Just tell me what's going on": The views of parents of children with genetic conditions regarding the research use of their child's electronic health record. J Am Med Inform Assoc 2020;27:429-36. [Crossref] [PubMed]
- Ajana B. Digital health and the biopolitics of the Quantified Self. Digit Health 2017;3:2055207616689509. [Crossref] [PubMed]
- All-Party Parliamentary Group on Sickle Cell and Thalassaemia. No one’s listening: an inquiry into the avoidable deaths and failures of care for sickle cell patients in secondary care. Published November 2021; accessed December 15, 2022. Available online: https://www.sicklecellsociety.org/wpcontent/uploads/2021/11/No-Ones-Listening-Final.pdf
- Middleton A, Milne R, Almarri MA, et al. Global Public Perceptions of Genomic Data Sharing: What Shapes the Willingness to Donate DNA and Health Data? Am J Hum Genet 2020;107:743-52. [Crossref] [PubMed]
- Platt J, Kardia S. Public trust in health information sharing: implications for biobanking and electronic health record systems. J Pers Med 2015;5:3-21. [Crossref] [PubMed]
- Aitken M, de St Jorre J, Pagliari C, et al. Public responses to the sharing and linkage of health data for research purposes: a systematic review and thematic synthesis of qualitative studies. BMC Med Ethics 2016;17:73. [Crossref] [PubMed]
- Atkin C, Crosby B, Dunn K, et al. Perceptions of anonymised data use and awareness of the NHS data opt-out amongst patients, carers and healthcare staff. Res Involv Engagem 2021;7:40. [Crossref] [PubMed]
- Brall C, Berlin C, Zwahlen M, et al. Public willingness to participate in personalized health research and biobanking: A large-scale Swiss survey. PLoS One 2021;16:e0249141. [Crossref] [PubMed]
- Trinidad MG, Platt J, Kardia SLR. The public’s comfort with sharing health data with third-party commercial companies. Humanit Soc Sci Commun 2020;7:149. [Crossref] [PubMed]
- Jones RD, Krenz C, Gornick M, et al. Patient Preferences Regarding Informed Consent Models for Participation in a Learning Health Care System for Oncology. JCO Oncol Pract 2020;16:e977-90. [Crossref] [PubMed]
- Grundstrom C, Korhonen O, Väyrynen K, et al. Insurance Customers' Expectations for Sharing Health Data: Qualitative Survey Study. JMIR Med Inform 2020;8:e16102. [Crossref] [PubMed]
- Weng C, Hao T, Friedman C, et al. Crowdsourcing Public Opinion for Sharing Medical Records for the Advancement of Science. Stud Health Technol Inform 2019;264:1393-7. [PubMed]
- Miyamoto SW, Henderson S, Young HM, et al. Tracking Health Data Is Not Enough: A Qualitative Exploration of the Role of Healthcare Partnerships and mHealth Technology to Promote Physical Activity and to Sustain Behavior Change. JMIR Mhealth Uhealth 2016;4:e5. [Crossref] [PubMed]
- Moon LA. Factors influencing health data sharing preferences of consumers: A critical review. Health Policy Technol 2017;6:169-87. [Crossref]
- Satinsky EN, Driessens C, Crepaz-Keay D, et al. Mental health service users' perceptions of data sharing and data protection: a qualitative report. J Innov Health Inform 2018;25:239-42. [PubMed]
- O'Brien EC, Rodriguez AM, Kum HC, et al. Patient perspectives on the linkage of health data for research: Insights from an online patient community questionnaire. Int J Med Inform 2019;127:9-17. Erratum in: Int J Med Inform 2020;136:104084. [Crossref] [PubMed]
- Iliadis A, Russo F. Critical data studies: an introduction. Big Data Soc 2016;1-7. [Crossref]
- Nafus D. Biosensing in Context: Health Privacy in a Connected World. In: Nafus D. Quantified: Biosensing Technologies in Everyday Life. MIT Press; 2016:79-100.
- Vidgen ME, Kaladharan S, Malacova E, et al. Sharing genomic data from clinical testing with researchers: public survey of expectations of clinical genomic data management in Queensland, Australia. BMC Med Ethics 2020;21:119. [Crossref] [PubMed]
- Haeusermann T, Fadda M, Blasimme A, et al. Genes wide open: Data sharing and the social gradient of genomic privacy. AJOB Empir Bioeth 2018;9:207-21. [Crossref] [PubMed]
- Darquy S, Moutel G, Lapointe AS, et al. Patient/family views on data sharing in rare diseases: study in the European LeukoTreat project. Eur J Hum Genet 2016;24:338-43. [Crossref] [PubMed]
- Luo Y, Oh CY, Jean BS, et al. Interrelationships Between Patients' Data Tracking Practices, Data Sharing Practices, and Health Literacy: Onsite Survey Study. J Med Internet Res 2020;22:e18937. [Crossref] [PubMed]
- Mählmann L, Schee Gen Halfmann S, von Wyl A, et al. Attitudes towards Personal Genomics and Sharing of Genetic Data among Older Swiss Adults: A Qualitative Study. Public Health Genomics 2017;20:293-306. [Crossref] [PubMed]
- Lucero RJ, Kearney J, Cortes Y, et al. Benefits and Risks in Secondary Use of Digitized Clinical Data: Views of Community Members Living in a Predominantly Ethnic Minority Urban Neighborhood. AJOB Empir Bioeth 2015;6:12-22. [Crossref] [PubMed]
- Mamo LA, Browe DK, Logan HC, et al. Patient informed governance of distributed research networks: results and discussion from six patient focus groups. AMIA Annu Symp Proc 2013;2013:920-9. [PubMed]
- Mazor KM, Richards A, Gallagher M, et al. Stakeholders' views on data sharing in multicenter studies. J Comp Eff Res 2017;6:537-47. [Crossref] [PubMed]
- Abdelhamid M, Gaia J, Sanders GL. Putting the Focus Back on the Patient: How Privacy Concerns Affect Personal Health Information Sharing Intentions. J Med Internet Res 2017;19:e169. [Crossref] [PubMed]
- Medford-Davis LN, Chang L, Rhodes KV. Health Information Exchange: What do patients want? Health Informatics J 2017;23:268-78. [Crossref] [PubMed]
- McCormack P, Kole A, Gainotti S, et al. 'You should at least ask'. The expectations, hopes and fears of rare disease patients on large-scale data and biomaterial sharing for genomics research. Eur J Hum Genet 2016;24:1403-8. [Crossref] [PubMed]
- Jagsi R, Griffith KA, Sabolch A, et al. Perspectives of Patients With Cancer on the Ethics of Rapid-Learning Health Systems. J Clin Oncol 2017;35:2315-23. [Crossref] [PubMed]
- Kalkman S, van Delden J, Banerjee A, et al. Patients' and public views and attitudes towards the sharing of health data for research: a narrative review of the empirical evidence. J Med Ethics 2022;48:3-13. [Crossref] [PubMed]
- Stranneheim H, Lagerstedt-Robinson K, Magnusson M, et al. Integration of whole genome sequencing into a healthcare setting: high diagnostic rates across multiple clinical entities in 3219 rare disease patients. Genome Med 2021;13:40. [Crossref] [PubMed]
- van der Velde KJ, Singh G, Kaliyaperumal R, et al. FAIR Genomes metadata schema promoting Next Generation Sequencing data reuse in Dutch healthcare and research. Sci Data 2022;9:169. [Crossref] [PubMed]
- Bell SA, Tudur Smith C. A comparison of interventional clinical trials in rare versus non-rare diseases: an analysis of ClinicalTrials.gov. Orphanet J Rare Dis 2014;9:170. [Crossref] [PubMed]
Cite this article as: Leigh S, Baines R, Stevens S, Garba-Sani Z, Austin D, Chatterjee A. Walk a mile in my shoes: perspectives towards sharing of health and experience data among individuals living with sickle cell disorder. mHealth 2024;10:4.



